Recibido: 31 de mayo de 2021; Aceptado: 18 de agosto de 2021
Abstract
Measuring interaction strength is key to understand the dynamics of mutualistic networks. However, how intraspecific variation within species traits can affect the patterns and outcomes of an interaction has been poorly measured. In this study, we explored how individual variation in fruit production in a fig tree species influences fruit consumption by frugivorous birds. Degree, expressed as the number of bird species, and visitation rates, expressed as number of individuals and its equivalent biomass, were independent of crop size. However, the cumulative number of visits for the three variables mentioned above was proportional to crop size. The number of small bird species (<100 g) was twice that from large species. However, the biomass of both groups was equivalent. Fruit consumption, expressed both as intake rate and total intake, was proportional to bird body mass. Our results suggest that the interaction between birds and fig trees depends on the size distribution of both organisms and the forest successional stage. In addition, from the consumers’ perspective, the amount of energy that each bird obtains depends on individual tree characteristics.
Key words:
Andes, Birds, Bird body size, Interaction strength, Resource availability..Resumen
Medir la fuerza de la interacción es clave para comprender la dinámica de las redes mutualistas. Sin embargo, la variación intraespecífica en los rasgos de las especies puede afectar los patrones y resultados de las interacciones a una escala aún no medida. En este estudio, exploramos cómo la variación individual en la producción de frutos en una especie de Ficus modula el consumo de frutos por aves. El grado, expresado como número de especies, y las tasas de visitas, expresadas como número individuos y su equivalente en biomasa, fueron independientes del tamaño de la cosecha. Sin embargo, el número acumulado para las tres variables fue proporcional al tamaño de la cosecha. El número de especies de aves pequeñas (<100 g) fue el doble que el de las especies grandes. Sin embargo, la biomasa de ambos grupos fue equivalente. El consumo, expresado tanto en tasa como en ingesta total, fue proporcional al tamaño corporal de las aves. Estos resultados sugieren que la interacción entre aves y Ficus depende de la distribución del tamaño de ambos organismos y de la etapa de sucesión del bosque. Además, desde la perspectiva de los consumidores, la cantidad de energía que obtiene cada ave depende de las características individuales del árbol.
Palabras clave:
Andes, Aves, Tamaño corporal de aves, Fuerza de interacción, Suministro de recursos..Introduction
Plant-animal mutualistic networks consist of sets of plants and animals that obtain reciprocal benefits from their interaction. In plant-frugivore networks, animals such as birds and mammals obtain food by consuming fruits, and during this process remove and disperse seeds (Bascompte & Jordano, 2014). A key aspect of network dynamics is the strength of the interaction, which reflects the influence that species have on each other (Vázquez et al., 2015; Wootton & Emmerson, 2005). Ultimately, this influence will represent an impact on the population dynamics of the interacting species (Vázquez et al., 2015). However, the interaction occurs among individuals of each species; thus, indices of interaction strength can be obtained at this level (Wootton & Emmerson, 2005).
Interaction strength is usually evaluated through interaction frequency. Nevertheless, factors related to the ecology of the interacting species, such as relative abundance, spatiotemporal distribution of individuals, and animal behavior, may modulate the interaction outcome in ways that are not captured by a simple frequency measurement (Palacio et al., 2015; Vázquez et al., 2009). Downscaling networks (i.e., exploring patterns of interaction at the individual level) is necessary to properly understand how intraspecific variation affects the interaction outcome, but few studies have explored such variation in detail. Downscaled networks show a structure that resembles the heterogeneity in specialization levels observed in interspecific networks, such as nestedness and asymmetry (Cantor et al., 2013). Variation in the interaction at the individual and functional group levels has been observed in both plant and animal mutualists (Benítez-Malvido et al., 2016; Cantor et al., 2013; Guerra et al., 2017; Palacio et al., 2015). For instance, the diet (plant species and item composition) of young and juvenile black howler monkeys (Alouatta pigra) is a nested subset of the adult diet (Benítez-Malvido et al., 2016).
The outcome of the plant-frugivore interaction may be asymmetric from the plant and the animal perspectives. From the plant’s perspective, different frugivorous species vary in their effectiveness as seed dispersers, depending on various aspects of their ecology and behavior (Schupp et al., 2010). The set of frugivores that visit an individual plant varies according to plant characteristics, such as fruit crop size, and factors related to the plant’s context, and this influences seed removal rates (Guerra et al., 2017; Palacio et al., 2015). For example, in the Neotropical treelet Miconia irwinii, a generalist species whose fruits are consumed by a broad range of bird species, variation at the individual plant level in fruit crop size and fruiting neighborhood affects different aspects of the interaction with seed dispersers, such as visitation and fruit removal rates (Guerra et al., 2017). Intraspecific variation in the quality and quantity of the interaction of a plant with different partner species results in variable effectiveness in the services provided by mutualists (Ness et al., 2006).
One factor that may affect the plant-frugivore interaction is the body size of the consumer species. In a variety of resource-consumer systems, consumption rates scale with consumer body mass with a power law (Brose et al., 2008; Rall et al., 2012). The scaling relationships describing measures of consumption rates as a function of predator size depend on the ecosystem and metabolic types of consumers (invertebrates versus vertebrates, ectotherms versus endotherms; Rall et al., 2012). Consumer body size is an important factor in the quantitative component of seed dispersal effectiveness (Martin, 1985; Schupp et al., 2010), but its role in structuring ecological networks has been little explored (Woodward et al., 2005). To our knowledge, no study has formally evaluated the relationship between food consumption and body size for frugivorous animals. This relationship is relevant from the plant’s perspective, because it may affect the number of seeds dispersed (Schupp et al., 2010). On the other hand, if food intake is proportional to body size, from the animals’ perspective, the benefit that frugivore species obtain may also depend on the individual plant they are visiting, but this aspect has been little studied (Vázquez et al., 2015).
In this research, we explore how intraspecific variation in fruit production in a montane species of fig tree (Ficus americana subsp. andicola), influences the pattern of interactions with different species in the frugivorous bird assemblage, and how this, in turn, influences rates of fruit removal. From the birds’ point of view, we explore how fruit crop size interacts with bird body size to determine fruit consumption rates and the energy intake that the birds obtain from an individual plant.
Materials and methods
This study was conducted between November 2003 and November 2004 in an area of about 100 ha within a 411 ha protected area, the Otún-Quimbaya Flora and Fauna Sanctuary. This reserve is adjacent to Ucumarí Natural Regional Park that protects an additional 4000 ha of cloud forest in the central range of the Colombian Andes, between 1750 and 2600 m a.s.l. Our study site ranged in elevation from 1800 to 2000 m a.s.l. The precipitation regime in the area is bimodal, with peaks in April and October and a mean annual rainfall of 2650 mm.
During the study period, we made observations on 13 Ficus americana subsp. andicola trees that produced fruit. For each tree, we estimated total crop size by estimating the number of fruits in a visible branch and extrapolating them to the entire tree, assuming that fruit production was similar among branches. We dried samples of fruits until constant mass, and extrapolated to the whole tree to express crop size as dry fruit biomass (g). Descriptive aspects of F. a. subsp. andicola’s biology are presented in Kattan & Valenzuela (2013). For each tree, we made a variable number of observation sessions of 4 hours. Crop duration (days) depended on crop size (number of fruits). Small crops (hundreds of fruits) were depleted fast (about 5 days) whereas large crops (>20 000 fruits) lasted 20 days or more. Thus, we made a number of observation sessions per tree (on different days) that was proportional to crop size (R2 = 0.33, P = 0.04, N = 13). In each observation session, we recorded the species of birds visiting the tree, number of individuals per species, consumption rate (number of fruits ingested per individual), and duration of visit in minutes. We compiled the information of bird body mass from published sources (e.g. Dunning, 2007).
From the interaction matrix, we calculated two network metrics, the overall degree of specialization (H´2) and nestedness (NODF), to understand whether this downscaling network behaves similarly to plant-frugivorous networks. Values of H´2 are scaled to range from 0 to 1, indicating the extremes of generalization and specialization, respectively. To identify non-random patterns of interactions between disperser species and plant individuals, we estimated nestedness for the individual-based network, using the NODF metric (Almeida-Neto et al., 2008) in ANINHADO (Guimarães & Guimarães, 2006). NODF values ranged from 0 (non-nested) to 100 (perfectly nested). We tested the significance of the observed value of nestedness through 1000 simulated networks generated by the Null Model II (Bascompte et al., 2003).
We used General Linear Models (GLM) to explore the degree (number of species) and visitation rates (individuals per observation session and biomass) as a function of crop size (fig fruits dry biomass). Then we used the Akaike Information Criterion (AIC) to select the best error distribution (Poisson, negative-binomial or normal) for each response variable. Also, having in mind that power-law relationships are one of the most common patterns in biology, and that they describe how almost any measurable characteristic of animals and plants scales with size (Brown et al., 2004), we also included within the different models linear regressions with transformed data to the natural logarithm.
We also expressed visitation rates as bird biomass, calculated by multiplying the number of individuals of each bird species in each observation session by mean body mass; this variable was also regressed against crop size. In addition, we obtained total consumer visitation (accumulated number of bird species, individuals, and biomass overall observation sessions) for each tree, and regressed it against crop size. For analyzing a total number of individuals, we divided birds into two size classes, defined as species smaller and larger than 100 g (there was a natural gap in size distribution between 60 and 130 g). We also calculated the biomass of consumers for the two bird size classes, by multiplying the number of individuals by the mean species body mass. We compared the total number of individuals and total biomass for the two bird size classes, regressed against crop size, with an analysis of covariance, taking into consideration that the best model fits a linear function with the transformed data.
To evaluate fruit consumption as a function of bird body mass, we analyzed mean fruit consumption rates per individual and total fruit intake per species, also using natural log-transformed variables and considering the different distribution options for the errors. For the first analysis, we expressed consumption rate (mean number of fruits per individual per min across all observation sessions) as a function of mean body mass for each bird species. For the second analysis, we multiplied mean fruit consumption rates (fruits.ind-1.min-1) by the duration in minutes of feeding bouts and mean number of individuals across all observation sessions. This variable represents the total amount of fig biomass consumed by each bird species per observation session (fruits.session-1). All analyses were generated with R software version 4.0 (R Development Core Team, 2020).
Results
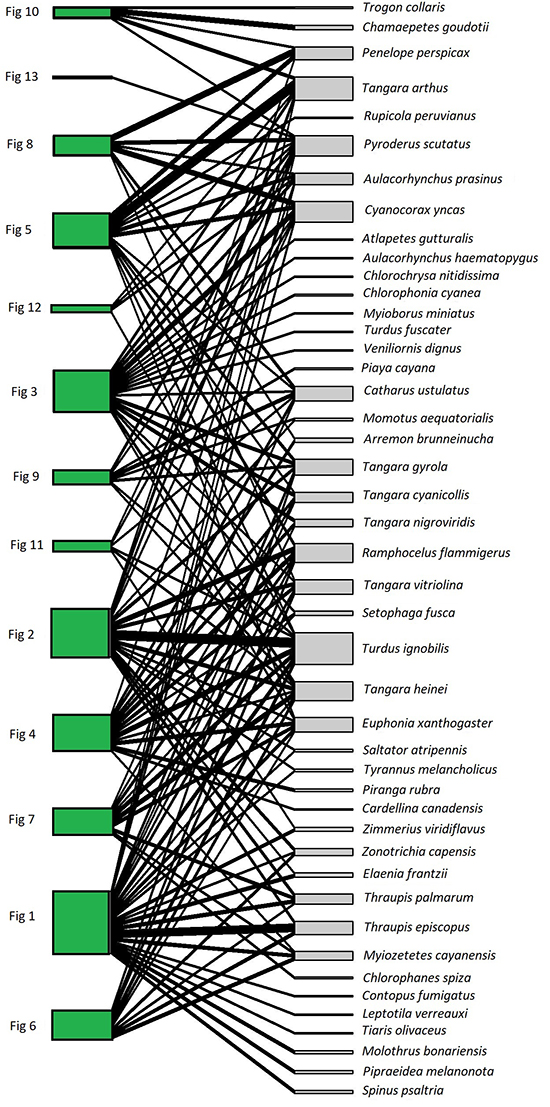
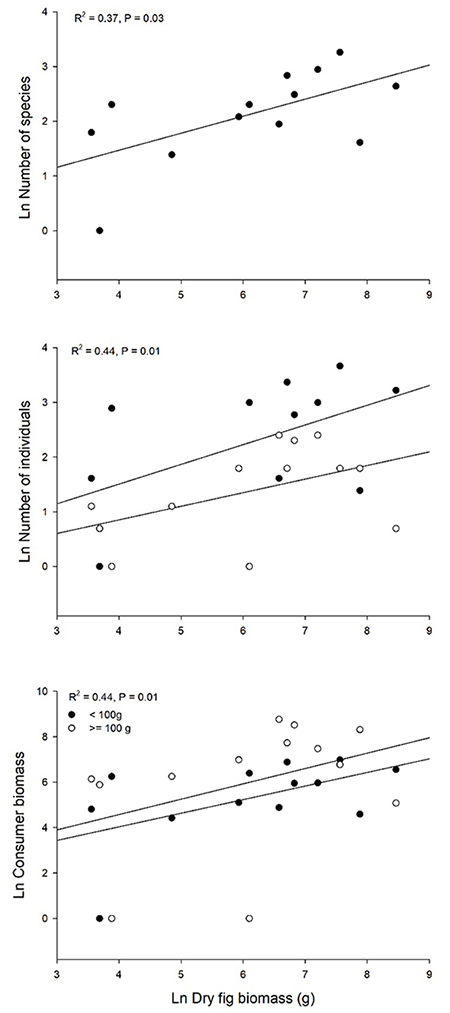
We recorded a total of 139 interactions between 13 plant individuals of F. a. subsp. andicola and 45 frugivorous bird species (Figure 1). The observed value of network-derived specialization (H´2) was 0.25. We found that the mutualistic network was significantly nested (observed matrix, NODF = 44.75; simulated matrices, NODF = 31.91; P < 0.05). Bird visitation rates to fig trees per 4-h observation session were independent of crop size (g dry fruit biomass), when expressed as the number of individuals (R2 = 0.19, P = 0.13) or biomass (R2 = 0.09, P = 0.3), like the number of species (R2 = 0.06, P = 0.41). In contrast, total bird visitation and bird species (accumulated number of visits across observation sessions) was proportional to crop biomass in all cases (Figure 2): total number of bird species (R2 = 0.37, P = 0.03), individuals (two size classes together, R2 = 0.44, P = 0.01) and biomass (R2 = 0.44, P = 0.01). For all relationships evaluated, the data fit the best to power-law models (Table S1).
Figure 1: Individual-based network of Ficus andicola subsp. andicola and its avian frugivores, in the Otún-Quimbaya Flora and Fauna Sanctuary, Risaralda, Colombia. The 13 sampled individuals of Ficus are on the left side, and the 45 bird species on the right side. The width of links and nodes corresponds to the intensity of the interaction.
Figure 2: Total bird visitation (feeding bouts) of Ficus andicola subsp. andicola, in the Otún-Quimbaya Flora and Fauna Sanctuary, Risaralda, Colombia, as a function of crop size, expressed as dry fruit biomass (g) per tree, accumulated across the observation sessions. Graphs show the number of species, number of individuals and bird biomass of fruit consumers for 13 crops. For number of individuals and biomass, separate curves are shown for the two size classes.
There were no significant differences between the slopes of the two bird size classes, for both total number of individuals (F1,23 = 0.27, P = 0.6, N = 13) and biomass (F1,23 = 0.018, P = 0.9, N = 13) when regressed against crop size. The intercept for number of individuals was higher for smaller birds (<100 g) than for larger birds (F1,23 = 7.06, P = 0.015, N = 13); on average there were 9.6 individuals of the small size class versus 4 individuals of the large size class. In contrast, although the mean biomass of small species was 197.3 g, and that of large species was 398.6 g, there was no significant difference in the intercept for total biomass of consumers for the two size classes as a function of crop size (F1,23 = 0.65, P = 0.43, N = 13).
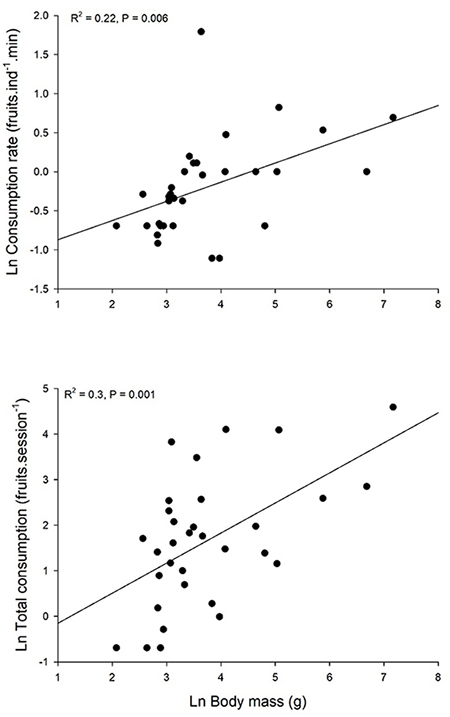
Fruit consumption rate (fruits.min-1) was proportional to consumer body size (R2 = 0.22, P = 0.006; Figure 3). Total fruit intake (fruits.session-1) for each bird species was also dependent on mean body mass (R2 = 0.3, P = 0.001; Figure 3). The slope of total energy intake as a function of body size was 0.66, i.e., total fruit intake was proportional to W2/3 based on the power-law model that in all cases showed the lowest AIC value (Table S1).
Figure 3: Fruit consumption of Ficus andicola subsp. andicola fruits in the Otún-Quimbaya Flora and Fauna Sanctuary, Risaralda, Colombia, as a function of bird body mass, in 4-hour observation sessions. Graphs show individual consumption rates and total consumption for each bird population (N = 32 bird species).
Discussion
At our study site, bird visitation rates per time unit were independent of fig tree crop size, even though crops varied between <1000 and >20 000 fruits. This suggests that there is a limited pool of consumers that can locate fruiting trees in a short period. However, larger crops remain available for a longer time and more birds in the regional pool could locate the trees, leading to the accumulation of a larger number of species and foraging visits. For other fig species, it has also been reported that the number of bird visitors is proportional to crop size (Sanitjan & Chen, 2009). Like other seed dispersal networks in nature, the figs network shows a low degree of specialization (0.25 Vs 0.23 for 8 networks, Blüthgen et al., 2007), suggesting that the efficiency of seed dispersal does not depend on specific dispersers and could be favored by a wide range of conditions (e.g., different body size, behavior, dispersal quality and habitat preferences) (Wheelwright & Orians, 1982; Blüthgen, 2007). The level of nestedness observed in our research is also similar to that reported in other plant-animal networks (Bascompte et al., 2003), including individual seed dispersal ones (Guerra et al., 2017).
We did not mark birds individually, so we may be counting the same individuals that keep coming back in different observation sessions. However, the fact that the total number of species increases with time (i.e., number of observation sessions on different days), suggests that different individuals are coming to feed to the trees. This is a mutualistic interaction, so the implications of this pattern from the tree point of view are important. Our results suggest that the different bird species are not equivalent as seed dispersers. Larger birds such as toucans and cracids, remove a high proportion of seeds in each feeding bout, while smaller birds such as tanagers and thrushes, remove lower numbers of seeds per visit. In general terms the assemblage of small seed dispersers is more diverse. This probably results in seeds being deposited in more varied habitats and at different distances from the parent tree, since different species of birds exhibit different behaviors and movement patterns (Wheelwright & Orians, 1982).
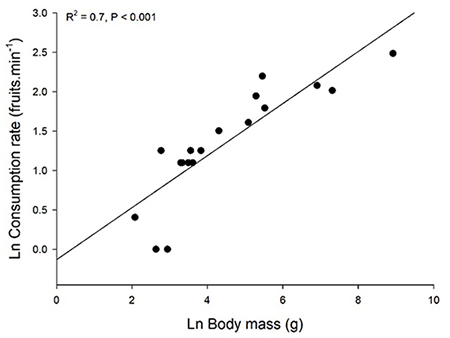
We found that both individual fruit consumption rates (fruits per time unit) and total fig intake of birds increased with body size. An increase in feeding rates with consumer body size was also reported for avian consumers of the tree Schefflera morototoni in Panama (Martin, 1985). Several studies have reported data on feeding rates and total consumption at fig trees but did not analyze the relationship with consumer body size (Coates-Estrada & Estrada, 1986; Goodman et al., 1997; Hamada & Hanya, 2016), probably because the number of consumer species in these studies was low. However, we plotted data reported in Coates-Estrada and Estrada (1986), which includes 14 bird species and 4 mammals consuming Ficus aff. cotinifolia in Mexico (Figure 4). For that species, both fig consumption rate and total consumption increased with consumer body mass.
The four mammal species are the largest in this assemblage and they fall within the same regression for birds. Thus, the pattern seems to hold independently of the consumer taxonomic group.
Figure 4: Relation of body mass and fruit consumption of Ficus aff. cotinifolia by 14 bird and 4 mammal species (the 4 largest body sizes) in Los Tuxtlas, México. Data plotted from Coates-Estrada & Estrada (1986).
As the fruit supply of F. a. subsp. andicola increased, the number of individuals of the two bird size classes increased in parallel, such that the accumulated biomass was similar for both. Does this translate into a similar rate of fruit consumption for both size classes at the population level? The higher number of individuals in the small size class was not sufficient to compensate for the higher rate of fruit consumption of large birds, so the total fruit intake increased with body size (Figure 3). Our results suggest that large body size confers a competitive advantage and larger bird species secure a larger share of resources. The mechanism may be simply related to fruit handling capacity: large birds have a large gape and can swallow several whole fruits in a single bout, whereas intermediate-sized birds may take one or two fruits, and the smallest ones can only take bites off a single fruit.
Our study adds to a mounting body of evidence that shows that intraspecific variation in individual traits affects the strength of the interaction between plants and fruit consumers (Benítez-Malvido et al., 2016; Cantor et al., 2013; Guerra et al., 2017). The interaction between fig trees and bird consumers depends on both tree crop size and bird body size (Emmerson & Raffaelli, 2004). As fig trees grow larger and produce more fruit (Kattan & Valenzuela, 2013), the assemblage of consumers changes; trees are visited by more and larger species. Thus, the distribution of sizes in the tree population and the bird assemblage is an important factor determining the global outcome of the interaction. Such ontogenetic changes have the potential to affect the dynamics of ecological networks (Woodward et al., 2005). From the birds’ perspective, the amount of energy that each species obtains also depends on the characteristics of the individual tree. On the other side, the dependence on large consumer species also reveals a vulnerability, as larger bird species are also more prone to be extirpated by environmental changes, with negative consequences for the network (Palacio et al., 2016).