Recibido: 27 de septiembre de 2020; Aceptado: 2 de marzo de 2021
Abstract
Chocó-Darien is an important biogeographic realm, as it is a terrestrial biodiversity hotspot and the southern limit of the Caribbean reefs. However, to date there are no compiled data on the reef fish assemblage of this region. We provide an updated checklist of marine fishes from the Chocó-Darien reef system (Colombia), with comments on their geographic distribution and conservation status. Peer-reviewed studies, unpublished data and in situ visual censuses were surveyed to compose this checklist. A total of 212 reef fish species across 57 families were compiled, eight of which had no previously published records, one of which (Trachinotus falcatus) is recorded for the first time. The most speciose families were Labridae (n = 21), Gobiidae (n = 18) and Serranidae (n = 17). Fourteen threatened species were recorded, including one critically endangered (Epinephelus striatus) and two endangered (Balistes vetula and Scarus coelestinus). This study contributes to fill the knowledge gaps on the reef fish diversity of the Caribbean southern limit and raises concern on the spread of the lionfish invasion into the Chocó-Darién reef system.
Keywords:
Atlantic, Biodiversity, Chocó-Darién, Neotropic, Pterois volitans..Resumen
El Chocó-Darién es una importante área biogeográfica, pues es un hotspot de biodiversidad terrestre y el límite sur de los arrecifes del Caribe. Sin embargo, hasta la fecha no existen datos compilados actualizados sobre las especies de peces de arrecife de esta región. En este trabajo proporcionamos una lista actualizada de peces marinos del sistema arrecifal del Chocó-Darién (Colombia), con comentarios sobre su distribución geográfica y estado de conservación. Para la elaboración de esta lista se consultaron publicaciones, datos no publicados y censos visuales in situ. Se recopilaron 212 especies de peces de arrecife de 57 familias. Ocho de estas especies no tenían registros previos publicados, y una de ellas es registrada por primera vez (Trachinotus falcatus). Las familias con mayor número de especies fueron Labridae (n = 21), Gobiidae (n = 18) y Serranidae (n = 17). Se registraron 14 especies amenazadas, entre ellas una en peligro crítico (Epinephelus striatus) y dos en peligro (Balistes vetula y Scarus coelestinus). Este estudio contribuye a complementar las lagunas de conocimiento sobre la diversidad de los peces de arrecife del límite sur del Caribe y plantea la preocupación de la invasión del pez león en el sistema arrecifal del Chocó-Darién.
Palabras clave:
Atlántico, Biodiversidad, Chocó-Darién, Neotrópico, Pterois volitans..Introduction
The continental coast of the southernmost portion of the Caribbean Sea, called Urabá Gulf, harbours particular ecological and geological features that differ markedly from other widely explored areas of the Caribbean (O´Dea, 2012). About 3.5 Myr ago, the area functioned as a deep ocean corridor connecting the fauna of the eastern Pacific and Caribbean Sea. After the closure of the Isthmus of Panama, two ecologically divergent areas were formed, with the Pacific side characterized by ocean-based environments and the Caribbean side dominated by coral reef ecosystems (Glynn, 1982).
This southernmost portion of the Caribbean Sea belongs to the Chocó-Darién biogeographic realm. It is regarded as a hotspot of global biodiversity, with high biological relevance for the Colombian Caribbean (Myers et al., 2000). The region encompasses large remnants of humid forest and a mosaic of coastal habitats, including riverine, estuarine and reef ecosystems (Díaz et al., 2000). Although the coastline is strongly influenced by large discharge of terrigenous sediment and freshwater from the Atrato River (Chevillot et al., 1993), fringing patch reefs flourish in such harsh conditions with a typical reef fish fauna and the largest living coral cover across the region (Díaz et al., 2000).
Despite its importance, the Chocó-Darién reef system remains poorly studied (but see Acero & Garzón, 1987a; Reyes-Nivia et al., 2004), with a limited knowledge on its ichthyofauna when compared to other areas of the Colombian Caribbean, such as the San Andrés and Providencia archipelago (Victoria & Gómez, 1984; Mejía & Garzón-Ferreira, 2000), Santa Marta (Acero & Garzón, 1987b; Acero & Rivera, 1992; Grijalba-Bendeck et al., 2004) and Islas del Rosario archipelago (Acero & Garzón, 1985; Delgadillo-Garzón & Zapata-Ramírez, 2009). Like other areas of the Colombian Caribbean, the Chocó-Darién reef has shown clear signs of degradation, evidenced by the dominance of macroalgae, pollution, unregulated tourism and, more recently, the occurrence of one of the invasive species of lionfish Pterois volitans (Betancur-R. et al., 2011; Gómez-López et al., 2018).
Lionfishes (P. miles and P. volitans) were the first non-native marine species to be established in the north-western North Atlantic to the Caribbean and Campeche Bank (Schofield, 2009). These species are considered voracious predators coupled with a high reproductive output (Côté et al., 2013). Hence, declines of native fish populations in reef systems have been correlated with the increment of their abundances (Green et al., 2012). Lionfishes have sharply dispersed across the Caribbean coral reefs after the first record at the Providencia Island, Colombia in 2008 (Betancur-R. et al., 2011). Over the last years, P. volitans has increasingly been reported at the Chocó-Darién reefs (Galvis & Galvis, 2016; García & Rueda 2018; Rojas-Vélez et al., 2019), which raises concern about the potential ecological outcomes in the near future.
This study provides an updated checklist of reef fish of the Chocó-Darién region, based on compiled data of visual censuses and literature, with notes on conservation status and species distribution. In addition, the current status on lionfish (P. volitans) invasion is discussed here. This compilation lays foundation for supporting ongoing and future studies for conservation of reef fishes and management of marine resources and services of the region.
Material and methods
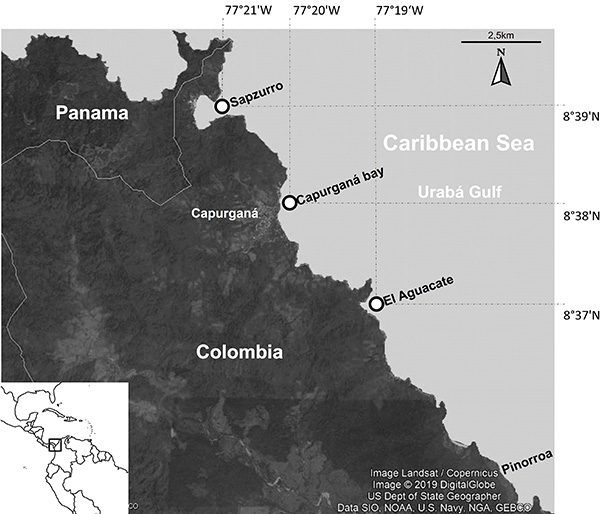
Study area. The study region is located in the western tip of the coast of the Urabá Gulf, Colombia, the southernmost portion of the Caribbean Sea. This area includes three sampling localities: Capurganá Bay (8°38’13.45”N; 77°20’39.29”W), El Aguacate (8°37’5.86”N; 77°19’28.53”W) and Sapzurro (8°39’37.02”N; 77°21’40.37”W) (Figure 1).
Figure 1: Western Caribbean coast of Colombia where the samplings of reef fish were carried out.
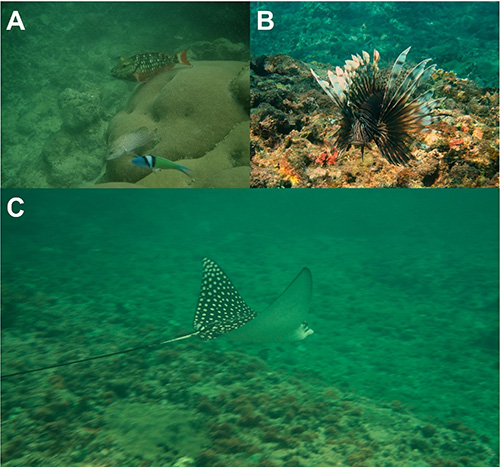
The waters of the Urabá Gulf are affected by an overload of freshwater and sediments from the Atrato River, which plays a critical role for local biogeography (Restrepo & Kjerfve, 2004). The Chocó-Darién reef system is characterized by a mosaic of fringing reefs, mainly composed by large colonies of Siderastrea siderea (Figure 2a) and a less extensive coral cover of Porites porites, Pseudodiploria strigosa, Agaricia agaricites, Agaricia tenuifolia and Millepora complanata (Gómez-López et al., 2018).
Figure 2: A small representation of the reef fish diversity recorded for the Chocó-Darién reef system, Colombia. A, Sparisoma viride, Thalassoma bifasciatum and Cephalopholis cruentata over a colony of Siderastrea siderea coral at El Aguacate; B, P. volitans at the Capurganá bay, ca. 7 m depth; C, Aetobatus narinari at the Capurganá bay.
Checklist compilation. The checklist was compiled from three sources: visual censuses in field expeditions, literature survey of peer-reviewed articles, and unpublished data (dissertations/theses).
Six visual censuses were performed, using roving diving technique (snorkeling) to quantify reef fish species richness in the Capurganá coastal reefs on April 2011 (Hill & Wilkinson, 2004). Three fringing coastal reefs were chosen, based on a previous survey and information on reef spatial arrangement (Díaz et al., 2000).
In the sampling sites, two divers freely swam for 30 minutes, identifying the fishes and, when possible, recording images and videos. The distance from the observer to the fish was a maximum of three meters, depending on the transparency, to avoid possible taxonomic errors. New records were only considered when two divers recorded the same species, or when a high-resolution image was available. Identifications of species in photos and videos were confirmed by comparisons with those provided by Reef Fish Identification Guide (Humann & Deloach, 2003).
The list of bony fishes was organized based on Eschmeyer’s Catalog of Fishes (Van der Laan et al., 2021), except for Labridae, in which Scarinae was included (Westneat & Alfaro, 2005), whereas Weigmann (2016) was followed for elasmobranchs. Genera and species are listed in alphabetical order. For each species included in the list, we confirm the geographic distribution based on Fishbase (Froese & Pauly, 2019). We also included the conservation status according to the Colombian National Red Book of marine fishes (Chasqui et al., 2017).
Results
Species list. A total of 212 reef fish species were compiled (Table 1), with 68 species recorded by the visual census. Eight out of the 212 species (3.7 %) had no previous published records: Coryphopterus dicrus, Dactylopterus volitans, Diodon holocanthus, Epinephelus adscensionis, Epinephelus guttatus, Ginglymostoma cirratum, Hypanus americanus, and Trachinotus falcatus. The latter species is a new record for the area and was sighted in our visual censuses. Additionally, we report a species of the Mugil curema complex (Nirchio et al., 2017) (Table 1).
Table 1: Species of reef fishes recorded for the Chocó-Darién reef ecosystem, Colombia. References: 1, Acero & Garzón (1987a) ; 2, Reyes-Nivia et al. (2004); 3, Peláez de la Torre (2010); 4, Guzmán & Leal (2011); 5, Ramírez & Gaviria (2013); 6, this study. Geographic range: CG, circumglobal; CT, Circumtropical; PAC, Pacific and Transatlantic (Appearing in both Western Atlantic and Eastern Atlantic); TA+MED, Transatlantic and Mediterranean;WA, Western Atlantic [Bahamas, Florida (USA), and northern Gulf of Mexico to Brazil]; WCA, Western Central Atlantic (Greater Caribbean); WI, Western Atlantic and Oceanic islands (Islands of St. Helena and Ascension). Conservation categories (IUCN 2019): CR, Critically Endangered; DD, Data deficient; EN, Endangered; LC, Least concern; NE, Not evaluated; NT, Near Threatened; VU, Vulnerable.
Species
Reference
Geographic range
Conservation Status
ORDER ORECTOLOBIFORMES
Family Ginglymostomatidae
Ginglymostoma cirratum (Bonnaterre 1788)
3, 4
TA
VU
ORDER MYLIOBATIFORMES
Family Dasyatidae
Hypanus americanus (Hildebrand & Schroeder 1928)
3, 6
WA
NT
Styracura schmardae (Werner 1904)
1
WA
NE
Family Myliobatidae
Aetobatus narinari (Euphrasen 1790)
1, 6
TA
NE
ORDER ANGUILLIFORMES
Family Muraenidae
Echidna catenata (Bloch 1795)
1, 2, 3, 6
WI
NE
Enchelycore carychroa Böhlke & Böhlke 1976
1
WI
NE
Enchelycore nigricans (Bonnaterre 1788)
1
TA
NE
Gymnothorax funebris Ranzani 1839
1, 4, 5
WA
NE
Gymnothorax miliaris (Kaup 1856)
1, 2, 3
TA
NE
Gymnothorax moringa (Cuvier 1829)
1, 4, 5
WI
NE
Gymnothorax vicinus (Castelnau 1855)
1
TA
NE
Family Chlopsidae
Kaupichthys hyoproroides (Strömman 1896)
1
CT
NE
Family Ophichthidae
Ahlia egmontis (Jordan 1884)
1
WA
NE
Myrichthys ocellatus (Lesueur 1825)
1
TA
NE
Family Moringuidae
Moringua edwardsi (Jordan & Bollman 1889)
1
WCA
NE
ORDER CLUPEIFORMES
Family Engraulidae
Anchoa lyolepis (Evermann & Marsh 1900)
1
WA
NE
Anchoviella perfasciata (Poey 1860)
1
WCA
NE
Family Clupeidae
Harengula clupeola (Cuvier 1829)
1
WA
NE
Jenkinsia lamprotaenia (Gosse 1851)
1
WCA
NE
Opisthonema oglinum (Lesueur 1818)
1
WA
NE
ORDER AULOPIFORMES
Family Synodontidae
Synodus intermedius (Spix & Agassiz 1829)
1, 2, 3
WA
NE
Synodus synodus (Linnaeus 1758)
1
TA
NE
ORDER HOLOCENTRIFORMES
Family Holocentridae
Holocentrus adscensionis (Osbeck 1765)
1, 2, 3, 4, 5
TA
NE
Holocentrus rufus (Walbaum 1792)
2, 3, 4, 5
WA
NE
Myripristis jacobus Cuvier 1829
1, 2, 3, 4, 6
TA
NE
Neoniphon marianus (Cuvier 1829)
2
WA
NE
Neoniphon vexillarium (Poey 1860)
1, 2, 3
WA
NE
Plectrypops retrospinis (Guichenot 1853)
1
WCA
NE
ORDER OPHIDIIFORMES
Family Ophidiidae
Parophidion schmidti (Woods & Kanazawa 1951)
1
WA
NE
Family Bythitidae
Ogilbia cayorum Evermann & Kendall 1898
1
WCA
NE
ORDER SCOMBRIFORMES
Family Scombridae
Scomberomorus regalis (Bloch 1793)
2
TA
NE
ORDER SYNGNATHIFORMES
Family Aulostomidae
Aulostomus maculatus Valenciennes 1841
1, 2, 3, 4, 6
WA
NE
Family Fistulariidae
Fistularia tabacaria Linnaeus 1758
4, 5
TA
NE
Family Syngnathidae
Cosmocampus brachycephalus (Poey 1868)
1
WA
NE
Halicampus crinitus (Jenyns 1842)
1
WCA
NE
Hippocampus reidi Ginsburg 1933
1
WA
VU
Microphis brachyurus (Bleeker 1854)
1
CT
NE
Family Dactylopteridae
Dactylopterus volitans (Linnaeus 1758)
5
TA+MED
NE
ORDER GOBIIFORMES
Family Gobiidae
Barbulifer ceuthoecus (Jordan & Gilbert 1884)
1
WA
NE
Bathygobius soporator (Valenciennes 1837)
1
TA
NE
Coryphopterus dicrus Böhlke & Robins 1960
3, 6
WA
NE
Coryphopterus eidolon Böhlke & Robins 1960
2
WCA
NE
Coryphopterus glaucofraenum Gill 1863
1, 2, 3
WA
NE
Coryphopterus lipernes Böhlke & Robins 1962
2
WCA
NE
Coryphopterus personatus (Jordan & Thompson 1905)
2, 3, 6
WCA
NE
Coryphopterus thrix Böhlke & Robins 1960
2, 3, 6
WCA
NE
Elacatinus illecebrosus (Böhlke & Robins 1968)
1, 2, 3, 6
WCA
NE
Elacatinus sp.
4, 5
Ginsburgellus novemlineatus (Fowler 1950)
1
WCA
NE
Gnatholepis thompsoni Jordan 1904
1, 2, 3, 6
TA
NE
Lythrypnus nesiotes Böhlke & Robins 1960
1
WCA
NE
Lythrypnus sp.
1
Lythrypnus spilus Böhlke & Robins 1960
1
WCA
NE
Priolepis hipoliti (Metzelaar 1922)
1
WA
NE
Tigrigobius multifasciatus (Steindachner 1876)
1
WCA
NE
Tigrigobius saucrus (Robins 1960)
2, 3
WCA
NE
ORDER CARANGIFORMES
Family Sphyraenidae
Sphyraena barracuda (Edwards 1771)
1, 3
CG
NT
Family Bothidae
Bothus lunatus (Linnaeus 1758)
1, 2, 3
TA
NE
Family Carangidae
Caranx bartholomaei Cuvier 1833
1, 2, 3
WI
NE
Caranx hippos (Linnaeus 1766)
1, 2, 4
TA+MED
VU
Caranx ruber (Bloch 1793)
1, 3, 4, 5, 6
WA
NE
Decapterus sp.
2
Trachinotus falcatus (Linnaeus 1758)
6
WA
NE
ORDER BELONIFORMES
Family Belonidae
Strongylura sp.
1, 6
ORDER MUGILIFORMES
Family Mugilidae
Mugil gr. curema
6
CG
NE
ORDER GOBIESOCIFORMES
Family Gobiesocidae
Acyrtops beryllinus (Hildebrand & Ginsburg 1927)
1
WA
NE
Acyrtus rubiginosus (Poey 1868)
1
WCA
NE
Gobiesox punctulatus (Poey 1876)
1
WA
NE
ORDER BLENNIIFORMES
Family Tripterygiidae
Enneanectes altivelis Rosenblatt 1960
1
WA
NE
Enneanectes boehlkei Rosenblatt 1960
1
WCA
NE
Family Labrisomidae
Gobioclinus kalisherae (Jordan 1904)
1
WA
NE
Labrisomus nuchipinnis (Quoy & Gaimard 1824)
1
TA
NE
Malacoctenus macropus (Poey 1868)
1
WCA
NE
Malacoctenus triangulatus Springer 1959
1, 2
WI
NE
Paraclinus nigripinnis (Steindachner 1867)
1
WA
NE
Starksia variabilis Greenfield 1979
1
WCA
NE
Stathmonotus gymnodermis Springer 1955
1
WA
NE
Family Chaenopsidae
Acanthemblemaria rivasi Stephens 1970
2, 3
WCA
NE
Coralliozetus sp.
1
Ekemblemaria nigra (Meek & Hildebrand 1928)
1
WCA
NE
Lucayablennius zingaro (Böhlke 1957)
2, 3
WCA
NE
Family Dactyloscopidae
Dactyloscopus tridigitatus Gill 1859
1
WA
NE
Platygillellus rubrocinctus (Longley 1934)
1
WCA
NE
Family Blenniidae
Entomacrodus nigricans Gill 1859
1
WCA
NE
Hypsoblennius invemar Smith-Vaniz & Acero P. 1980
1
WA
NE
Ophioblennius macclurei (Silvester 1915)
1, 3, 4, 6
WA
NE
ORDER ACANTHURIFORMES
Family Pomacanthidae
Holacanthus ciliaris (Linnaeus 1758)
1, 2, 4
WI
NE
Holacanthus tricolor (Bloch 1795)
2, 4
WA
NE
Pomacanthus arcuatus (Linnaeus 1758)
1, 2, 3, 4, 6
WA
NE
Pomacanthus paru (Bloch 1787)
1, 2, 3, 4, 6
WI
NE
Family Chaetodontidae
Chaetodon capistratus Linnaeus 1758
1, 2, 3, 4, 5, 6
WA
NE
Chaetodon ocellatus Bloch 1787
1, 2, 3, 4, 5, 6
WA
NE
Chaetodon sedentarius Poey 1860
2, 6
WA
NE
Chaetodon striatus Linnaeus 1758
2, 3, 4, 5, 6
WI
NE
Family Acanthuridae
Acanthurus chirurgus (Bloch 1787)
1, 2, 3, 4, 5, 6
TA
NE
Acanthurus coeruleus Bloch & Schneider 1801
1, 2, 3, 4, 5, 6
TA
NE
Acanthurus tractus Poey 1860
1, 2, 3, 4, 5, 6
WCA
NE
ORDER LOPHIIFORMES
Family Antennariidae
Antennarius multiocellatus (Valenciennes 1837)
1
WI
NE
ORDER TETRADONTIFORMES
Family Diodontidae
Diodon holocanthus Linnaeus 1758
4
CT
NE
Diodon hystrix Linnaeus 1758
1, 2, 3, 6
CT
NE
Family Tetraodontidae
Canthigaster rostrata (Bloch 1786)
1, 2, 3, 4, 6
WCA
NE
Sphoeroides spengleri (Bloch 1785)
1
WA
NE
Sphoeroides testudineus (Linnaeus 1758)
1
WA
NE
Family Ostraciidae
Acanthostracion polygonius Poey 1876
2, 3
WA
NE
Acanthostracion quadricornis (Linnaeus 1758)
2, 3
TA
NE
Lactophrys bicaudalis (Linnaeus 1758)
2, 3, 6
WI
NE
Lactophrys trigonus (Linnaeus 1758)
1
WA
NE
Lactophrys triqueter (Linnaeus 1758)
2, 3
WA
NE
Family Monacanthidae
Aluterus scriptus (Osbeck 1765)
2, 3
CT
NE
Cantherhines macrocerus (Hollard 1853)
2, 3, 4
WI
NE
Cantherhines pullus (Ranzani 1842)
1, 2, 3, 4, 6
TA
NE
Monacanthus tuckeri Bean 1906
2
WCA
NE
Family Balistidae
Balistes vetula Linnaeus 1758
2
TA
EM
Canthidermis sufflamen (Mitchill 1815)
1, 2, 3, 6
TA
NE
Melichthys niger (Bloch 1786)
2
CT
NE
ORDER CENTRARCHIFORMES
Family Kyphosidae
Kyphosus sp.
2, 3, 6
Kyphosus sectatrix (Linnaeus 1758)
1, 4, 5
TA+MED
NE
Family Cirrhitidae
Amblycirrhitus pinos (Mowbray 1927)
2, 3
WA
NE
ORDER ACROPOMATIFORMES
Family Pempheridae
Pempheris schomburgkii Müller & Troschel 1848
1, 2, 3, 4, 5, 6
WA
NE
ORDER PERCIFORMES INCERTAE SEDIS
Family Serranidae
Cephalopholis cruentata (Lacepède 1802)
1, 3, 4, 5, 6
WCA
NE
Cephalopholis fulva (Linnaeus 1758)
1, 3
WA
NE
Epinephelus adscensionis (Osbeck 1765)
4, 5
TA
NE
Epinephelus guttatus (Linnaeus 1758)
4, 5
WA
NT
Epinephelus striatus (Bloch 1792)
1, 2, 3, 6
WA
CR
Hypoplectrus nigricans (Poey 1852)
2, 3
WCA
NE
Hypoplectrus puella (Cuvier 1828)
1, 2, 3, 4, 5, 6
WCA
NE
Hypoplectrus unicolor (Walbaum 1792)
2, 3
WCA
NE
Liopropoma rubre Poey 1861
2
WA
NE
Mycteroperca bonaci (Poey 1860)
1, 2
WA
VU
Mycteroperca tigris (Valenciennes 1833)
2
WA
NT
Mycteroperca venenosa (Linnaeus 1758)
2
WA
VU
Pseudogramma gregoryi (Breder 1927)
1
WA
NE
Rypticus saponaceus (Bloch & Schneider 1801)
1, 2, 4, 5
TA
NE
Rypticus subbifrenatus Gill 1861
1
TA
NE
Serranus baldwini (Evermann & Marsh 1899)
1
WA
NE
Serranus tigrinus (Bloch 1790)
1, 2, 3, 4, 5, 6
WCA
NE
Family Grammatidae
Gramma loreto Poey 1868
1, 2, 3, 4, 5, 6
WA
NE
Gramma melacara Böhlke & Randall 1963
2
WCA
NE
Family Opistognathidae
Opistognathus whitehursti (Longley 1927)
1
WA
NE
Family Priacanthidae
Heteropriacanthus cruentatus (Lacepède 1801)
2, 3
CT
NE
Family Apogonidae
Apogon maculatus (Poey 1860)
1
WA
NE
Astrapogon puncticulatus (Poey 1867)
1
WA
NE
Phaeoptyx conklini (Silvester 1915)
1
WA
NE
Phaeoptyx pigmentaria (Poey 1860)
1
TA
NE
Family Malacanthidae
Malacanthus plumieri (Bloch 1786)
1
WI
NE
Family Lutjanidae
Lutjanus analis (Cuvier 1828)
1, 2, 3, 6
WA
VU
Lutjanus apodus (Walbaum 1792)
2, 3, 4
WA
NE
Lutjanus cyanopterus (Cuvier 1828)
1, 2
WA
VU
Lutjanus griseus (Linnaeus 1758)
1
WA
NE
Lutjanus jocu (Bloch & Schneider 1801)
1, 2, 3
TA
DD
Lutjanus mahogoni (Cuvier 1828)
1, 2, 3, 4
WCA
NE
Lutjanus synagris (Linnaeus 1758)
2, 3
WA
NE
Ocyurus chrysurus (Bloch 1791)
1, 2, 3, 4, 5, 6
TA
NT
Family Gerreidae
Eucinostomus sp.
1
Gerres cinereus (Walbaum 1792)
1
WA
NE
Family Haemulidae
Anisotremus surinamensis (Bloch 1791)
2, 3
WA
NE
Anisotremus virginicus (Linnaeus 1758)
1, 2, 3, 4, 6
WA
NE
Brachygenys chrysargyreum (Günther 1859)
2, 3
WA
NE
Haemulon album Cuvier 1830
2, 3
WA
LC
Haemulon aurolineatum Cuvier 1830
1, 2, 3, 4, 6
WA
NE
Haemulon bonariense Cuvier 1830
2, 3
WCA
NE
Haemulon carbonarium Poey 1860
1, 2, 3, 4, 6
WCA
NE
Haemulon flavolineatum (Desmarest 1823)
1, 2, 3, 4, 6
WA
NE
Haemulon macrostoma Günther 1859
1, 2, 3, 4, 5, 6
WA
NE
Haemulon parra (Desmarest 1823)
2, 3
WA
NE
Haemulon plumierii (Lacepède 1801)
1, 2, 3, 6
WA
NE
Haemulon sciurus (Shaw 1803)
2, 3, 4, 5
WA
NE
Family Sciaenidae
Equetus lanceolatus (Linnaeus 1758)
2, 3
WA
NE
Equetus punctatus (Bloch & Schneider 1801)
2, 3, 4, 6
WA
NE
Odontoscion dentex (Cuvier 1830)
2, 3
WA
NE
Family Mullidae
Mulloidichthys martinicus (Cuvier 1829)
1, 2, 3, 4, 5, 6
TA
NE
Pseudupeneus maculatus (Bloch 1793)
1, 2, 3, 4, 5, 6
WA
NE
ORDER PERCIFORMES
Family Pomacentridae
Abudefduf saxatilis (Linnaeus 1758)
1, 2, 3, 4, 5, 6
TA+MED
NE
Abudefduf taurus (Müller & Troschel 1848)
1, 6
TA
NE
Chromis cyanea (Poey 1860)
2
WCA
NE
Chromis insolata (Cuvier 1830)
2
WCA
NE
Chromis multilineata (Guichenot 1853)
1, 2, 3, 4, 6
TA
NE
Microspathodon chrysurus (Cuvier 1830)
1, 2, 3, 4, 6
WA
NE
Stegastes adustus (Troschel 1865)
1, 2, 3, 4, 5, 6
WCA
NE
Stegastes diencaeus (Jordan & Rutter 1897)
2, 3
WCA
NE
Stegastes leucostictus (Müller & Troschel 1848)
1, 2, 3, 6
WA
NE
Stegastes partitus (Poey 1868)
1, 2, 3, 4, 6
WCA
NE
Stegastes planifrons (Cuvier 1830)
1, 2, 3, 4, 6
WCA
NE
Stegastes xanthurus (Poey 1860)
1, 2, 3
WCA
NE
Family Labridae
Bodianus rufus (Linnaeus 1758)
1, 2, 3, 4, 6
WA
NE
Clepticus parrae (Bloch & Schneider 1801)
2, 3, 4
WCA
NE
Doratonotus megalepis Günther 1862
1
TA
NE
Halichoeres bivittatus (Bloch 1791)
1, 2, 3, 4, 5, 6
WA
NE
Halichoeres cyanocephalus (Bloch 1791)
2
WA
NE
Halichoeres garnoti (Valenciennes 1839)
1, 2, 3, 4
WA
NE
Halichoeres maculipinna (Müller & Troschel 1848)
1, 2, 3, 4, 5, 6
WA
NE
Halichoeres pictus (Poey 1860)
2, 3
WCA
NE
Halichoeres poeyi (Steindachner 1867)
1, 2, 3, 6
WA
NE
Halichoeres radiatus (Linnaeus 1758)
1, 2, 3, 4, 5, 6
WI
NE
Scarus coelestinus Valenciennes 1840
2
WA
EM
Scarus iseri (Bloch 1789)
1, 2, 3, 6
WCA
NE
Scarus taeniopterus Lesson 1829
2, 3
WA
NE
Scarus vetula Bloch & Schneider 1801
2, 3
WCA
NT
Sparisoma atomarium (Poey 1861)
2, 6
WA
NE
Sparisoma aurofrenatum (Valenciennes 1840)
1, 2, 3, 4, 5, 6
WCA
NE
Sparisoma chrysopterum (Bloch & Schneider 1801)
2, 3, 6
WCA
NE
Sparisoma radians (Valenciennes 1840)
1
WA
NE
Sparisoma rubripinne (Valenciennes 1840)
1, 2, 3, 4, 5, 6
WA
NE
Sparisoma viride (Bonnaterre 1788)
1, 2, 3, 4, 5, 6
WA
NT
Thalassoma bifasciatum (Bloch 1791)
1, 2, 3, 4, 5, 6
WA
NE
Family Scorpaenidae
Pterois volitans (Linnaeus 1758)
4, 6
PAC
NE
Scorpaena bergii Evermann & Marsh 1900
1
WA
NE
Scorpaena grandicornis Cuvier 1829
1
WA
NE
Scorpaena isthmensis Meek & Hildebrand 1928
1
WA
NE
Scorpaena plumieri Bloch 1789
1, 4
WI
NE
Scorpaenodes caribbaeus Meek & Hildebrand 1928
1
WA
NE
Scorpaenodes tredecimspinosus (Metzelaar 1919)
1
WA
NE
Chocó-Darién reef fishes are distributed in 21 orders and 57 families, the most representative orders being Perciformes plus Perciformes incertae sedis (43.86 %), followed by Gobiiformes (8.49%), Blenniiformes (8.49 %), and Tetraodontiformes (8.02 %). The most speciose families were Labridae (9.91 %), followed by Gobiidae (8.49 %), Serranidae (8.02 %), Haemulidae and Pomacentridae (5.66 %). Likewise, the most speciose genera were Haemulon (n = 9), followed by Halichoeres and Lutjanus (n = 7), Sparisoma, Stegastes and Coryphopterus (n = 6).
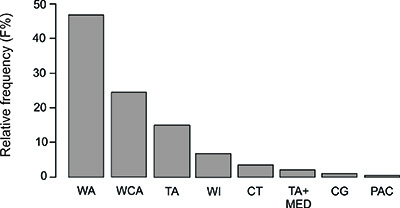
From the recorded fishes, 96 are widespread species of the Western Atlantic, 50 of the Greater Caribbean, 33 Trans-Atlantic, seven Circumtropical, four Transatlantic and Mediterranean, two Circumglobal and one restricted to the Pacific Ocean (Figure 3). The latter species is the introduced lionfish P. volitans (Figure 2b).
Figure 3: Relative frequency of the geographic range categories of reef fish recorded for the Chocó-Darién reef system, Colombia. CG, circumglobal; CT, circum-tropical; PAC, Pacific; TA, Transatlantic (Western Atlantic and Eastern Atlantic); TA+MED, Transatlantic and Mediterranean; WA, Western Atlantic; WCA, Western Central Atlantic (Caribbean); WI, Western Atlantic and Oceanic islands.
Of the 212 species, one is listed as Data Deficient (DD), one species as Least Concern (LC), seven as Near-threatened (NT), seven as Vulnerable (VU), two as Endangered (EN) and one as Critically Endangered (CR). A total of 10 species are in a threat category for the Chocó-Darién coral reef, based in the National Red Book (Chasqui et al., 2017). The Nassau grouper (Epinephelus striatus) is Critically Endangered. In addition, the Queen Triggerfish Balistes vetula and the Midnight Parrotfish Scarus coelestinus are categorized as Endangered. However, most species (186) have not yet been evaluated in Colombia.
Discussion
This work represents an extensive compilation on the ichthyofauna diversity of the Chocó-Darién reef system, Colombian Caribbean. Previous studies have documented contrasting number of fish species, such as 146 (Acero & Garzón, 1987a) and 119 (Reyes-Nivia et al., 2004), which is possibly due to the use of different sampling methodologies.
The most speciose families recorded in this work (e.g., Labridae, Gobiidae, Serranidae, Haemulidae and Pomacentridae) are commonly found in the continental margins of the tropical Atlantic (Floeter, 2008). Nevertheless, the fish richness recorded for the study area (212 species) represents only 30 % of the total species accounted across the Caribbean region, indicating its relatively low richness. For example, Acero & Garzon (1987 b) recorded 372 species at the Santa Marta reef systems, Colombian Caribbean, and Starck (1968) recorded 389 species at the Alligator reef, Florida Keys.
Recently, a biogeographic analysis for fish data of both reef and soft bottom divided the Caribbean region into three major provinces: (1) a central, tropical province comprising the West Indies, Bermuda and Central America; (2) a southern, upwelling-affected province spanning the entire continental shelf of northern South America; and (3) a northern, subtropical province that includes all of the Gulf of Mexico, Florida and south-eastern USA (Robertson & Cramer, 2014). The Chocó-Darién reef system is located at the southern province covering the entire continental shelf of northern South America, holding the lowest number of fish species and percentage of local endemics (3.4 %). Likely, the particular environmental conditions of this region, such as the high loads of nutrients, low pH, temperature and salinity variations caused by the Atrato River (McLaughlin et al., 2003, Manzello, 2010) explain, in part, the low species richness of the region.
Most of the listed species present distributions across the Western Atlantic. Biogeographically, the Western Atlantic comprises the Greater Caribbean and Brazil, with their faunas considered until recently to be partially separated by the freshwater discharge from the Amazon and Orinoco river mouths (Floeter et al., 2008). However, an extensive and diverse reef system was recently mapped for the Amazon region (called Great Amazon Reef), which represents the northern limit of the Brazilian Province and may function as an ecological corridor connecting the fauna of the Brazil and Caribbean (Francini-Filho et al., 2018). Importantly, the Caribbean shelters a higher species richness (774 species) and endemism levels (57 %) compared to Brazil (Kulbicki et al., 2013, Pinheiro et al., 2018), which, in turn, explains the high percentage of Caribbean or Western Central Atlantic endemics species in our study (23 %).
The presence of the species Gnatholepis thompsoni and Pterois volitans in the Chocó Darién region is related to different events. G. thompsoni, native from the Indian Ocean, possibly dispersed to the north Atlantic during the last interglacial period, and its expansion range has been spreading in face of climate change (Rocha et al., 2005). On the other side, P. volitans was introduced in the Florida Keys coastal waters in the 1980’s, as a consequence of escapes of the aquarium trade (Morris et al., 2009). Its range has increased over the years, reaching a broad extension of the tropical and subtropical Western Atlantic and Caribbean (Schofield, 2009; Betancur-R et al., 2011). In Colombia, this lionfish was firstly reported in the oceanic islands, and subsequently in the coastal areas of the Caribbean (Betancur-R. et al., 2011). Our study raises concerns of the species´ invasion at the Chocó-Darién reef system (Galvis & Galvis, 2016; Rojas-Vélez et al., 2019), and supports the previous reports of the expansion of P. volitans toward southernmost portion of the Caribbean Sea. This species feeds on a wide variety of juveniles of large-bodied fish (Green & Côté, 2014), and crustaceans, as already reported for Colombian Caribbean regions (Muñoz-Escobar & Gil-Agudelo, 2012; Acero et al., 2019). Such feeding behavior may trigger impacts on local fish populations, and consequently, in the food web dynamics (Valdez-Moreno et al., 2012).
The present study contributes to fill up the knowledge gap on reef fish of the Chocó-Darién reef system. Further research including new technologies such as ROVs (Auster, 1997) and baited remote underwater video systems (BRUVs) (White et al., 2013) are recommended, which would enable exploring remote reef areas, such as mesophotic and rariphotic ecosystems (cf. Francini-Filho et al., 2019). Finally, in face of the fast invasion of the P. volitans and the high occurrence of threatened fishes, we recommend strengthening fish monitoring programs to subsidize management and conservation measures at the Chocó-Darién reef system.