Recibido: 28 de mayo de 2020; Aceptado: 15 de noviembre de 2020
Abstract
Fish produce sounds that are usually species-specific and associated with particular behaviors and contexts. Acoustic characterization enables the use of sounds as natural acoustic labels for species identification. Males of Prochilodus magdalenae produce mating sounds. We characterized these sounds and tested their use in natural habitats, to use passive acoustic monitoring for spawning ground identification. We identified two types of acoustic signals: simple pulses and pulse trains. Simple pulses were 13.7 ms long, with peak frequency of 365 Hz, whereas pulse train were 2.3 s long, had peak frequency of 399 Hz, 48.6 pulses and its pulses lasted 12.2 ms, with interpulse interval of 49.0 ms long and 22.3 Hz pulse rate. We did not detect spawning in absence of male calls nor differences in male sounds at different female densities. We found differences in train duration, pulse rate, and pulse duration in trains, according to the fish’s source sites, but these sites were not well discriminated based on bioacoustical variables. In rivers, we located two P. magdalenae spawning grounds and recognized calls from another fish species (Megaleporinus muyscorum). We did not find a significant relationship between fish size and call peak frequency for P. magdalenae.
Keywords:
Bocachico, Potamodromous fish, Soniferous fish, Spawning grounds..Resumen
Los peces producen sonidos que generalmente están asociados con comportamientos y contextos particulares y son especie-específicos. Su caracterización permite usarlos como etiquetas acústicas naturales para identificar especies. Los machos de Prochilodus magdalenae emiten sonidos de apareamiento. Caracterizamos estas señales acústicas y probamos su uso en hábitats naturales, para utilizar el monitoreo acústico pasivo para la identificación de zonas de desove. Identificamos dos tipos de señales acústicas: pulsos simples y trenes de pulsos. Los pulsos simples tuvieron una duración de 13.7 ms y frecuencia pico de 365 Hz, mientras que los trenes de pulsos duraron 2.3 s, frecuencia pico de 399 Hz, 48.6 pulsos por tren, pulsos de 12.2 ms, intervalo interpulso de 49.0 ms y tasa de pulsos de 22.3 Hz. No detectamos desove en ausencia de sonidos de machos ni diferencias acústicas entre machos en parejas o en grupos de hembras. Dependiendo del origen de los machos, la duración del tren, frecuencia del pulso y duración del pulso en los trenes difirieron, pero los sitios no fueron bien discriminados basados en las variables bioacústicas. En ríos localizamos dos zonas de desove de P. magdalenae y pudimos distinguir sus sonidos respecto a los del pez Megaleporinus muyscorum. No encontramos una relación significativa entre el tamaño y la frecuencia pico para P. magdalenae.
Palabras clave:
Bocachico, Peces potamódromos, Peces que producen sonidos, Zonas de desove..Introduction
More than 700 species of fish around the world are known to produce sounds (Kaatz, 2002), of which at least 80 are freshwater fishes (Greenhalgh et al., 2020). However, this number is probably higher and it is estimated that a third of the total living fish species have the ability to emit this type of signal (Ladich & Bass, 2011). The sounds produced are generally species-specific and are associated with particular behaviors and contexts, including those related to reproductive displays (Amorim et al., 2015). Fish are the vertebrates that have evolved the largest diversity of sound-producing mechanisms, and these mechanisms are generally classified into two large groups: those based on rubbing of bony structures against each other and those based on the vibration of the swim bladder through specialized muscles (Ladich & Fine, 2006).
The characterization of acoustic signals has been carried out mainly for marine fish, but it has also been used for freshwater fish (Borie et al., 2014; Godinho et al., 2017; Greenhalgh et al., 2020). This characterization allows the use of sounds as natural acoustic labels for species identification, and can be made ex situ, or in situ. The ex situ approach has been widely used and validated (Rountree et al., 2006), as confined conditions allow better noise control, individual isolation of fish and more specificity in sound analysis.
A possible application of sound characterization in fish is location of natural spawning grounds through identification of acoustic signals associated with mating in natural conditions, by applying passive acoustic monitoring (PAM). Loss of these spawning grounds could lead to a lack of recruitment of new individuals in populations, so location of the areas used for spawning is important information for conservation and management of any migratory fish that spawns in rivers. (Godinho et al., 2017). . Hydropower development can lead to the loss of spawning grounds of migratory fish (Filho & Schulz, 2004), and in the case of Colombia, this development has been focused in Andean rivers, particularly in the Magdalena basin (Operman et al., 2015).
One species that is known to produce sounds associated with mating is the bocachico, Prochilodus magdalenae, a potamodromous freshwater migratory fish endemic to Colombia, distributed in all the lower areas of the Magdalena, Sinú and Atrato River basins, but reaching up to 1500 m. a.s.l. during its potamodromous upstream migrations in the Cauca River basin (Mojica et al., 2012). P. magdalenae is the main species of Colombia’s inland fisheries (Barreto & Mosquera, 2001), but currently it faces various anthropogenic threats jeopardizing its persistence in natural environments (Mojica et al., 2012).
Species of Prochilodus have been documented to produce sounds in the reproductive season, by the contraction of the first three or four intercostal muscles around the swim bladder, characterized by being hypertrophied and presenting an intensive red color (Harder, 1975; Godinho et al., 2017). Although mating calls by Prochilodus magdalenae males have been noted for many decades, their calling has not been characterized. To determine if PAM could be used as a method to locate spawning grounds over a long river reach for this species, we recorded and acoustically characterized male calls at fish hatchery stations located in three river systems; determined the importance of calls for spawning; tested if males call differentially if they were in groups, or just with a female; tested if males from different origins call distinctively; sampled for spawning calls of P. magdalenae at three river systems, and determined if it was possible to distinguish their calls from other sounds or fish calls recorded simultaneously in rivers.
Materials and methods
Bioacoustic characterization of fish calls in captivity. To record the calls, in June, August, and October 2018 we visited three fish stocking hatcheries in the Cauca, Sinú, and Magdalena Rivers, in Colombia. We recorded 47 sexually mature male and 75 female individuals of P. magdalenae from natural populations (born in rivers and kept in the fish stations) , in two categories: couples (one male with one female in one pond at a time) and group sets (one male with two or three females in one pond at a time). We recorded 16 couples from the Cauca River at the Centro Acuícola Piscícola Santa Cruz S.A.S, department of Antioquia; 19 males with 47 females (3 couples and 16 group sets) from the Sinú River, at the Centro de Investigaciones Piscícolas de la Universidad de Córdoba, department of Córdoba; and 12 couples from the Magdalena River at the Centro Piscícola San Silvestre S.A., department of Santander.
Each audio recording set consisted of a Song Meter SM4 bioacoustic recorder (Wildlife Acoustics), a HTI-96-Min Exportable hydrophone (High Tech Inc.) and a SM3 / SM4 H-1 inlet adapter ref 1094101 (Wildlife Acoustics). For each recording, we located a hydrophone at the middle of the pond, approximately 20 cm below water surface, and for every couple or group of P. magdalenae individuals. Recording was of 1 hour long, corresponding to half an hour before and half an hour after the fish estimated spawning time, according to the endocrine manipulation protocol used at each fish farm to induce fish spawning, because gravid females of Prochilodus magdalenae do not spontaneously spawn in captivity (Atencio, 2001). For each male individual we registered date and place of the recording, standard fish length (cm) for males, and water temperature (°C), measured with a YSI 550A equipment (YSI Inc.). After each recording, we checked for the presence of eggs with hand sieves to determine whether females spawned or not.
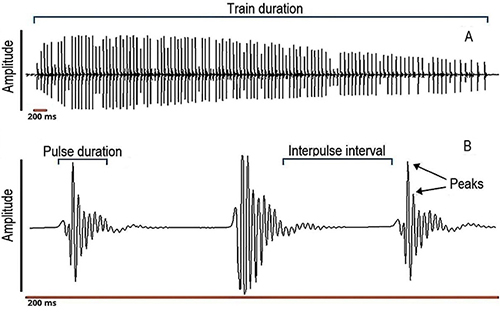
Prochilontids are known to emit low-frequency sounds; however, since we had no specific reference for Prochilodus magdalenae sound frequency, the sounds were digitized at a sampling rate of 12 000 Hz, to avoid possible information loss. All sound files were visualized and analyzed using oscillograms, spectrograms, and the pulse analysis tools of Avisoft SASLab Pro version software (v.5.2.07). We used a bandwidth of 235 Hz and a fast Fourier transformation (FFT) of 256 samples, frequency resolution of 47 Hz and a time overlap of 50 %. The sounds produced by Prochilodus were categorized into two types, using the definitions of Smith et al., (2018): simple pulses (or also called “isolated pulses”) and pulse trains (a series of at least two repetitive and stereotyped simple pulses), easily recognizable, considering the structuring of these sounds in other species of the genus. The simple pulses were recognized by their waveform in the oscillograms, also visible in pulses that were part of trains (Figure 1). Pulses were defined as those that had duration of less than 25 ms and were detected using the peak search with hysteresis method, that is based on a relative peak detection algorithm that identifies local peaks in the envelope signal. A peak is recognized when its peak amplitude exceeds the minimum amplitude that precedes this peak by a predefined factor, and is certainly useful for analyzing signals that could be influenced by reverberation (http://www.avisoft.com/SASLabPro.pdf). We analyzed the total length of all recordings.
Figure 1: Oscillogram of a pulse train, showing how train duration, pulse duration and interpulse interval were measured, and how peaks per pulse were counted. A, a complete train of pulses; B, magnification of the train representing 200 ms.
The bioacoustic variables we measured for simple pulses were: i) total number of simple pulses (count); ii) simple pulse duration (ms), as the time from the first upward to the last downward deflection of the pulse); iii) simple pulse peak frequency (Hz), as the frequency with the highest amplitude, also called dominant frequency. For pulse trains, the variables measured were: i) total number of trains (count); ii) total number of pulses in a train (count): iii) train duration (s) as the time between the start of the first pulse and the end of the last pulse in a train; iv) duration of pulses in train (ms), having the same definition as simple pulse duration; v) interpulse interval (the time from the end of one pulse to the beginning of the subsequent); vi) pulse rate (Hz) as the number of pulses occurring in a train of pulses, divided by the duration of that train (s) (Luczkovich et al., 2008); and vii) train peak frequency (Hz) as the mean peak frequency of all pulses within a train, according to literature (Colleye & Parmentier, 2012; Schärer et al., 2014; Smith et al., 2018). All these variables were measured for each individual male fish. We also included a power spectrum for a pulse train referenced to the root mean square (RMS) amplitude (3 dB below the peak amplitude) and also compared our results to those published for other species of Prochilodus.
The number of pulses and trains per individual were used for statistical analyses, following Smith et al. (2018) and Fine & Parmentier (2015), while for the remaining bioacoustic variables we estimated the mean values per individual, and with this data we elaborated a general reference table of bioacoustic characteristics of P. magdalenae calls, presenting mean or median values of the bioacoustical variables. Descriptive statistics was applied to all the data corresponding to each variable, as well as for checking the normality of all data and evaluating whether they met the variance homogeneity criterion, thus deciding whether to use parametric or non-parametric tests. The Shapiro-Wilk test (p<0.05) was used to inspect normality and the Levene test (p<0.05) was used for homogeneity of variance.
The importance of calls for spawning was determined by comparing the percentage of spawning occurrences in presence or absence of male calls. Statistical analyses were performed to test significant differences (p<0.05) in the bioacoustic variables between i) the individuals arrays, if the sound record corresponded to a couple (male accompanied by a single female) or to a “group” (if a male was recorded accompanied by more than one female): to test these differences we used Mann-Whitney U test or the T-Student’s test, depending to normality and homogeneity of variance; ii) the place of origin of the individuals (if their origin was the Cauca, Sinú or Magdalena River): to test these differences we used Kruskal-Wallis test, ANOVA or Welch test depending on the case, and in case of significant differences (p<0.05), we applied a post-hoc test (Dunn’s or Tukey’s tests) to identify such differences. A linear discriminant analysis for P. magdalenae males and their bioacoustical variables was applied to evaluate differences between river basins; for this analysis, we used the Paleontological Statistic Software PAST (v 4.03). We carried out Pearson’s correlation analyses to evaluate the strength of relationship between fish size (as standard length) and dominant frequency for simple pulses and pulse trains, and to evaluate temperature values in respect to each of the bioacoustical variables.
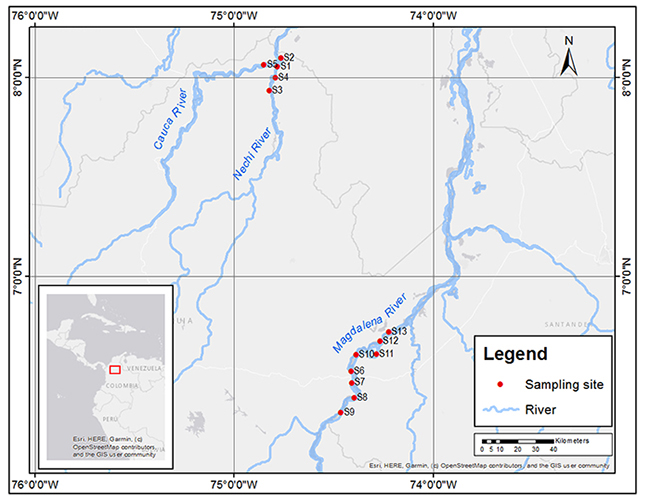
River Sampling. In April 2019, during the first fish spawning season of the year, we m ade 7 hours of audio recordings at 13 places recognized by artisanal fishers as historical sites for reproduction of P. magdalenae and other soniferous native fish species: eight sites in the Magdalena River, four sites in the Nechí River and one site in the Cauca River (Figure 2). We reached sampling sites by boat and geo-referenced them with a Garmin eTrex 20x GPS; the obtained coordinates were imported into ArcMap 10.2.2 to construct the location map of the sites where recordings were performed.
Figure 2: Sampling sites for audio recordings of Prochilodus magdalenae in the Nechí (S1-S4), Cauca (S5) and Magdalena (S6-S13) Rivers, in northern Colombia.
While making our recordings, the boat was moored and its motor was turned off. Recording length at each site was variable, depending on the distance. Due to security issues, all recordings were performed during daytime. We used the same methods of sound analysis as described for audio recordings of fish in captivity; however, since Prochilodus magdalenae makes “choruses” calls in nature, the analyses were focused on the sounds of interest detected at each sampling site, and not by individual fish. We applied, when possible, descriptive statistics (counts, central tendency and variability measurements) to the same bioacoustical variables as described for fish in captivity, and the results were compared to the general reference table elaborated with the bioacoustical data of captive fish. We also used the P. magdalenae bioacoustics reference table obtained with captive fish to help distinguish P. magdalenae calls from other fish acoustic signals recorded in the rivers at same time.
Animal welfare. The use and handling of specimens in this study followed the guidelines for institutional and national animal welfare and management; the survival of the specimens was not compromised, nor was any individual slaughtered.
Results
Bioacoustic characterization of calls of fish in captivity. We identified 1523 acoustic signals produced by 27 P. magdalenae males in 47 hours of recording in the three fish hatcheries: 1312 signals were isolated pulses and 211 were pulse trains. The mean standard length for specimens was 24.1 ± 4.3 cm. We could hear calls several meters away from the fishpond, showing that this species can make strong acoustic signals. Samples of these sounds are available in scientific archive collections (see supplementary material for details).
Considering all the individuals that made sounds, we found that simple pulse (Shapiro-Wilk, p<0.01, W=0.52) and train counts (Shapiro-Wilk test, p=0.04, W=0.90) were highly variable and did not follow a normal distribution, whereas for all other variables normality was met (Shapiro-Wilk test, p>0.05) (Table 1). P. magdalenae calls presented short pulse duration and low peak frequency for both simple pulses and pulse trains. Another characteristic of trains is that the interpulse interval duration is almost four times longer than duration of those simple pulses that constitutes trains with relative low variation, as well as low variation in the pulse rate of trains; on the other hand, pulse duration in trains is more variable. Train duration mean value was 2.3 s and the longest individual train signal we registered was 7.3 s in duration.
Table 1: Reference values for the bioacoustic variables in simple pulses (n=27) and pulse trains (n=20) of Prochilodus magdalenae kept in captivity in Colombia. Values correspond to the mean and the standard deviation (x̄ ± sd), except for simple pulse and train counts, for which the median is presented followed by quartiles one and three: Me (Q1; Q3).
Variable
Value
Simple pulse count (n)
7.0 (2; 45)
Simple pulse duration (ms)
13.7 ± 4.0
Simple pulse peak frequency (Hz)
365 ± 102
Train count (n)
8.5 (3; 20)
Simple pulse count in train (n)
48.6 ± 28.5
Train duration (s)
2.3 ± 1.2
Pulse duration in train (ms)
12.2 ± 6.0
Interpulse interval (ms)
49.0 ± 7.0
Pulse rate (Hz)
22.3 ± 1.5
Train peak frequency (Hz)
399 ± 144
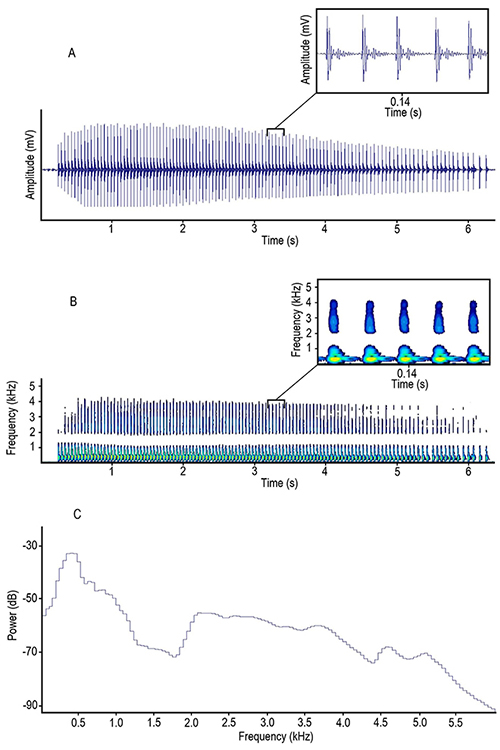
The waveforms of pulses were characterized by having two dominant peaks followed by several peaks whose amplitude gradually decreases in a damped oscillation pattern as shown in Figure 3-A. We found that trains were simple, characterized by a sequence of many similar pulses (Figure 3-A, B). In power spectrum the highest peak was at around 400 Hz and there was a second wider peak at around 2 to 3 kHz; then power dropped, presenting a gradual decrease as frequency values increased (Figure 3-C).
Figure 3: A, oscillogram, B, spectrogram and C, power spectrum of a pulse train emitted by Prochilodus magdalenae in Colombia. Yellowish areas in the magnified spectrogram section represent the highest amplitude levels in pulses.
No differences were detected in the bioacoustic characteristics of simple pulses between individuals of different rivers (Table 3), but there were some significant differences between individuals from different rivers in the duration and pulse rate of trains, as well as in the duration of train pulses. The duration of trains between individuals from the Sinú and Magdalena rivers was different, being longer for individuals of the Magdalena River. Fish from the Cauca River presented the highest pulse rate and the shortest duration of pulses in a train (Table 3). Water temperature in fishponds had a mean value of 28.9 ± 1.6 °C (n=27), and there were significant differences among the fish farms (p<0.05, F=12.06), being highest at the Cauca river fish farm.
Table 2: Bioacoustic variables of simple pulses and pulse trains of Prochilodus magdalenae males in couples and groups in Colombian hatcheries. Values correspond to the mean and the standard deviation (x̄ ± sd), except for simple pulse and train counts, for which the median is presented followed by quartiles one and three: Me (Q1; Q3). The means or medians that share the same superscript letter were not significantly different. Mann-Whitney U test α=0.05 and Student’s T test α=0.05.
Level
Statistics
Pair
Group
Simple pulse variables
(n)=23
(n)=4
Simple pulse count (n)
7.0 (2.5; 37.0)a
19.5 (1.0; 99.0)a
W=43 ; p=0.863
Simple pulse duration (ms)
14.0 ± 4.0a
11.00 ± 2.1a
T=-1.076 ; p=0.293
Simple pulse peak frequency (Hz)
379 ± 103a
296 ± 69a
T=-1.529 ; p=0.140
Pulse train variables
(n)=16
(n)=4
Train count (n)
11.50 (4.5; 19.3)a
2.0 (1; 5.8)a
W=13 ; p=0.079
Simple pulse count in train (n)
53.3 ± 28.7a
29.6 ± 20.5a
T=-1.541 ; p=0.141
Train duration (s)
2.5 ± 1.2a
1.3 ± 0.9a
T=-1.777 ; p=0.092
Pulse duration in train (ms)
11.0 ± 6.7a
15.0 ± 3.6a
T=1.012 ; p=0.325
Interpulse interval (ms)
50.0 ± 7.0a
49.0 ± 9.0a
T=-0.2456 ; p=0.809
Pulse rate (Hz)
22.4 ± 1.6a
21.7 ± 1.6a
T=-0.685 ; p=0.504
Train peak frequency (Hz)
420 ± 141a
313 ± 136a
T=-1.366 ; p=0.189
Table 3: Bioacoustic variables of simple pulses and pulse trains of Prochilodus magdalenae in Colombia, and water temperature at fish farms by river of origin of males. Values correspond to the mean and the standard deviation (x̄ ± sd), except for simple pulse and train counts, for which the median is presented followed by quartiles one and three: Me (Q; Q3). The means or medians that share the same superscript letter were not significantly different. ANOVA: α=0.05, Kruskal-Wallis test: α=0.05, Tukey test: α=0.05.
Level
Statistics
Sinú
Magdalena
Cauca
Simple pulse variables
(n)=7
(n)=8
(n)=12
Simple pulse count (n)
4.0 (1.0; 51.0)a
24.0 (9.5; 102.3)a
4.0 (2.0; 12.0)a
X
2= 2.989 ; p=0.224
Simple pulse duration (ms)
12.0 ± 2.0a
14.0 ± 4.0a
15.0 ± 5.0a
F=2.115 ; p=0.144
Simple pulse peak frequency (Hz)
316 ± 80a
341 ± 56a
424 ± 124a
F=3.069 ; p=0.067
Pulses train variables
(n)=6
(n)=6
(n)=8
Train count (n)
2.5 (1.3; 6.7 )a
7.5 (3.5; 12.3)a
19.0(6.8; 20.8)a
X
2=5.679 ; p=0.058
Simple pulse count in train (n)
26.9 ± 19.8a
61.1 ± 36.9a
55.3 ± 18.8a
F=3.113 ; p=0.070
Train duration (s)
1.3 ± 0.9a
3.1 ± 1.3b
2.4 ± 0.8ab
F=4.917 ; p=0.021
Pulse duration in train (ms)
16.7 ± 5.3a
14.7 ± 4.9 a
6.8 ± 3.9b
F= 8.762 ; p=0.002
Interpulse interval (ms)
48.6 ± 7.9a
55.8 ± 7.0a
46.0 ± 5.8a
F=2.759 ; p=0.095
Pulse rate (Hz)
21.6 ± 0.8a
21.0 ± 0.7ac
23.5 ± 1.2b
F=1.403 ; p=0.278
Train peak frequency (Hz)
327 ± 159a
356 ± 49a
485 ± 147a
F=2.7168 ; p=0.118
Physicochemical variable
(n)=7
(n)=8
(n)=12
Temperature (°C)
27.4 ± 0.3 a
28.2 ± 0.2 a
30.4 ± 1.2 b
F=12.06 ; p=0.00055
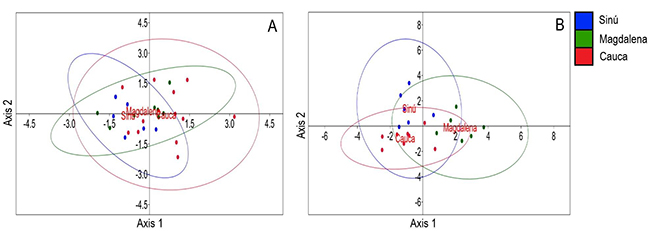
The river of origin of P. magdalenae males was not well discriminated by bioacoustical variables for both simple pulses and pulse trains, as there is high overlapping among groups. For simple pulses (Figure 4-A), the Axis 1 eigenvalue was 0.401 and explained 97.56 % of data and Axis 2 eigenvalue was 0.010 (2.44 %); for pulse trains (Figure 4-B) the Axis 1 eigenvalue was 2.622 and explained 70.25 % of the data and Axis 2 eigenvalue was 1.110 (29.75 %). Total number of simple pulses and trains were not included in this analysis (not normally distributed variables).
Figure 4: Linear discriminant analysis scores and 95 % confidence ellipses for Prochilodus magdalenae males and their bioacoustical variables, to evaluate differences between river origin of individuals. A, simple pulses (n=27) and B, pulse trains (n=20)
We found no significant linear relationship (Pearson’s correlation analysis, p>0.05) between P. magdalenae standard length and dominant frequency for both trains and single pulses. The dominant frequency was not related to fish size. In the same way, we found no significant correlation of any bioacoustical variable with temperature; although, the correlation between temperature and pulse duration in trains had the most relevant coefficient among all variables, it was still weak (R=-0.350), and not significant (p=0.13).
There was no occurrence of egg releases in the fishponds when no male sound was detected. Additionally, when only simple pulses were recorded, spawning happened in 51.9 % of ponds, and raised to 85.7 % when trains of pulses were registered.
River Sampling. We did not detect soniferous activity in the Nechí and Cauca rivers, while in the Magdalena River we identified soniferous activity at two sites: site S10, with 18 P. magdalenae calls, and site S6, with 6 calls; water temperatures were 25.2 and 26.41°C, respectively, for each site. These records consisted of multiple pulse trains emitted synchronically or “choruses”, that lasted from 12.8 s to 382.6 s (6.4 minutes), with a median duration of 29.6 s and quartiles Q1=18.8 s and Q2=53.6 s. Since P. magdalenae congregates for spawning, many males usually produce sounds simultaneously near each other, resulting in a cacophony. This made sound analysis very difficult and we could only analyze bioacoustically three good quality fragments in which there was no call overlap (at the beginning or end of choruses). We identified these calls as fragments of trains. We did not detect simple pulse signals in the recordings.
The mean peak train frequency values and mean pulse duration in train fragments, as well as its standard deviations, had values similar to what was found for trains in captive fish. However, the interpulse interval was shorter in the records of fish in rivers, reflecting a higher pulse rate than for fish in captivity. Bioacoustic variable values for both captive and fish are shown in Table 1 and Table 4. At sampling site S6 we also detected 11 conspicuous train signals different from those of P. magdalenae. Although we did not have a bioacoustical reference to match the characteristics of those signals, they were strong enough to be heard above water, and were identified by traditional fishers as belonging to Megaleporinus muyscorum, locally known as comelón or moino.Table 4 shows values for the bioacoustical variables of this species.
Table 4: Values of bioacoustic variables of Prochilodus magdalenae train fragments (n=3) and Megaleporinus muyscorum trains (n=11) in the Magdalena River, Colombia. Values correspond to the mean value and the standard deviation (x̄ ± sd).
Variable
P. magdalenae (Train fragment)
M. muyscorum (Train)
Simple pulse count (n)
9.7 ± 3.8
6.4 ± 2.8
Duration (s)
0.2 ± 0.1
0.7 ± 0.4
Pulse duration (ms)
16.0 ± 0.01
13.9 ± 0.001
Interpulse interval (ms)
10.0 ± 0.002
111.0 ± 0.02
Pulse rate (Hz)
45.2 ± 2.8
9.5 ± 1.3
Peak frequency (Hz)
373 ± 20
607 ± 61
Discussion
The mating calls of individuals of P. magdalenae kept at fish hatcheries were similar to vocalizations emitted by other species of Prochilodus (Godinho et al., 2017; Smith et al., 2018). They are also common to many fish species that produce sounds through vibratory muscles acting on the swim bladder, indicating that it is a conserved character related to this mechanism of sound production in fish (Fine & Parmentier, 2015).
The pulse trains of P. magdalenae are similar to those of P. argenteus, P. costatus and P. lineatus (see also Smith et al., 2018 for comparisons). There is a rapid raising of signal amplitude at the beginning of trains, reaching the maximum amplitude, and then there is a general gradual decrease in amplitude until the train ends. The most distinctive species is P. costatus, in which the decrease in amplitude after reaching maximum is more marked.
In all these species the shape of the pulses, including number of peaks per pulse, varied considerably by train; however, each pulse generally started with two dominant peaks, followed by a few subsequent peaks that gradually decrease in amplitude (Figure 3A; Smith et al., 2018). Moreover, power spectrum for trains of P. magdalenae share similarities with those described for P. argenteus, P. costatus and P. lineatus, -a characteristic gradual decrease in power as frequency values increased, and power of the calls was below 1 kHz, with peaks usually below 500 Hz (Smith et al., 2018).
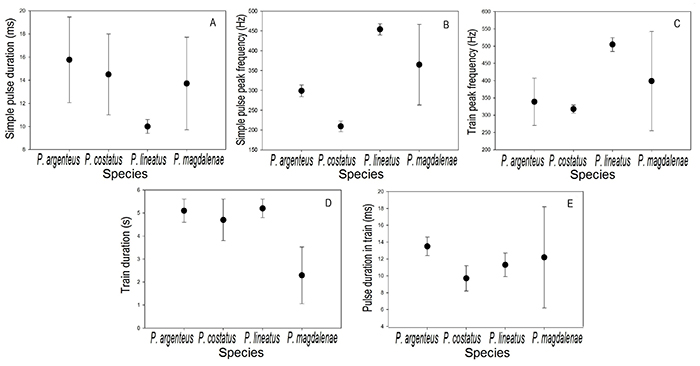
Simple pulses of P. magdalenae are shorter than those of P. costatus and P. argenteus, but longer than those reported for P. lineatus (Smith et al., 2018; Figure 5-A). However, there are overlaps between the pulse duration of these species, considering the intraspecific signal variation (standard deviations), indicating that this bioacoustic feature did not have a high degree of interspecific differentiation. The peak frequency of simple pulses in P. magdalenae was lower than in P. lineatus, but higher than in P. argenteus and P. costatus (Figure 5-B); P. argenteus, P. costatus and P. lineatus have clearly different peak frequency values and no overlaps between them, indicating that this variable is specific for these species when compared to each other, while for P. magdalenae, the results suggest that it has a wider spectrum of peak frequencies when emitting this type of acoustic signals.
The highest train peak frequency value was reported for P. lineatus, followed by P. magdalenae, while P. argenteus and P. costatus presented similar peak frequencies (Figure 5-C). On the other hand, the trains of P. magdalenae are the shortest of all species, whereas the other three species have similar train duration (Figure 5-D), despite the fact that P. argenteus and P. costatus are sympatric in most of their distribution range and they are sympatric with P. lineatus in rivers in which they have been introduced (Smith et al., 2018). In general, the four compared species emitted trains with relatively similar pulse duration. P. argenteus emitted the longest pulses, while P. costatus the shortest and P. magdalenae presented the greatest variation, compared to the three other species (Figure 5-E).
Figure 5: Reference values (● =mean; ⊢ = standard deviation) of the bioacoustic variables of Prochilodus magdalenae (n=27 for simple pulse variables and n=20 for pulse train variables) at 27.4-30.4°C, compared with data data available for P. argenteus (n=3) at 28-30 °C, P. costatus (n=3) at 28-30 °C, and P. lineatus (n=3) at 27 °C (Smith et al., 2018). A, simple pulse duration; B, simple pulse peak frequency; C, train peak frequency; D, train duration; E, pulse duration in train.
The fact that there were no significant differences between males that were in couples or in groups for any of the bioacoustic variables measured, for both simple pulses and pulse trains, suggests that these characteristics of male calls in this species are not influenced by the density of females during the reproductive event (Table 2). However, since the groups had a small sample size (n=4) these results must be taken with care.
The low-frequency sounds of these four Prochilodus species is typical in fish acoustic signaling associated to mating behavior (Amorim et al., 2015). Sound production in all these species is in the form of a series of pulses, which is dependent upon sonic muscles associated to the swim bladder, showing in general similar characteristics of the waveform for both trains and simple pulses and also similar characteristics in power spectrum. However, there can be enough differences to discriminate species at a bioacoustical base, as shown by Smith et al. (2018) for the prochilodontids they studied. The most distinctive bioacoustical feature of P. magdalenae was the shorter trains of pulses. Train duration, however, may not encode species-specific or even individual-specific information, but it might instead reflect individual motivational information (Smith et al. 2018).
The discriminant analysis results suggest that for P. magdalenae, calls are similar among different areas of the distribution range of this species, when considering all the bioacoustical variables used; simple pulse duration and peak frequency of pulses and trains of P. magdalenae were highly conserved acoustic characteristics, as they did not show differences in relation to grouping (social context) or provenance. Pulse trains, on the other hand, were different among origin rivers specifically in terms of train duration, pulse duration in train and pulse rate. Variation in acoustic signals at geographical level can be an adaptive response to the different acoustic characteristics of the environment in which they live.
Bioacoustical geographic differences can also be related to physiological responses to the immediate environment, such as water temperature, which may impact metabolism and muscle activation that affect the bioacoustic characteristics of sounds (Köhler et al., 2017). Considering that most fish (including P. magdalenae) are poikilothermic ectotherms, they lack physiological adaptations to thermoregulate, so water temperature is one of the most important extrinsic factors related to the muscular performance of these organisms. The work done by a muscle decreases significantly with decreasing temperature, while the speed and power increases with increasing temperature (James, 2013). Therefore, temperature differences in water can affect the rate of contraction of the intercostal muscles that produce trains of pulses. This could explain the shorter pulse duration and higher pulse rate of the captive fish in the Cauca River, and the shorter trains of captive fish of the Sinú River, as, on average, temperature at the Sinú was 3°C below that at the Cauca. However, the effect of temperature on sound duration in fish is complex (Ladich & Maiditsch, 2020); thus, for example, interpulse duration in P. magdalenae for individuals in rivers was shorter, although water temperature was lower than that of fish farms.
Larger individuals of fish usually produce lower frequency calls (Connaughton et al., 2000). However, frequency values that vary little in relation to size have been reported for some species (Fine, 1978), and this is probably also the case for Prochilodus (Smith et al., 2018). Fish species that produce sounds based on a high-speed muscle mechanism (as in Prochilodus), have minimal changes in peak frequency with respect to changes in size of individuals, because the frequency is dependent on the rate of muscle contraction and not so much on the size of the swim bladder (Parmentier & Fine, 2016).
The male calling behavior is a fundamental aspect that could trigger spawning in P. magdalenae. The relationship between sound production by males (especially trains) and spawning events by females provides key data that helps explain the functional meaning of these calls and their importance in the reproductive behavior of P. magdalenae. A role of male calls in spawning has been also found in other Prochilodus species (Godinho et al., 2017), and Fontenele (1953) noticed males calling while mating. However, since there were several males observed in that study, it is not certain if calling was only from the males engaged in mating. Playback experiments could be considered to clarify specifically the communicative component of the acoustic signal in P. magdalenae, with respect to other signals that might be related to reproduction, such as physical contact and possible visual cues between individuals.
Conclusions
PAM can be done to locate spawning grounds of P. magdalenae over a long river reach. This kind of monitoring can be a very potent capture-independent tool for studying this species, presenting new opportunities for developing more integral conservation strategies.
Peak frequency, and pulse duration can be used to identify P. magdalenae in natural environments. Additionally, by comparing interpulse interval, pulse rate and duration of the trains it is also possible to differentiate P. magdalenae from other species, such as M. muyscorum.
Other Colombian fish species, such as Curimata mivartii, Plagioscion magdalenae, Brycon moorei, Pseudoplatystoma magdaleniatum, which, according to artisanal fishers, produce sounds, could be studied with PAM. These species are also highly important for Colombians fisheries, so knowing them in greater detail will provide more elements for better management and conservation.
Through PAM, it will also be possible to study the migratory events of soniferous fish as well as the behaviors associated with the emission of acoustic signals. Therefore, more research at the captive level is required to systematically study these bioacoustic aspects. The knowledge obtained about the sounds of fish will allow automating the detection of species for multiple purposes in natural water bodies.
The audio files uploaded to the ML and IAvH-CSA sound collections can be used for different research purposes and for divulgation-educational programs, increasing in this way the knowledge and awareness about these species, apporting new tools for studying and protecting them. This approach is applicable for other soniferous fish species reported in Colombia, yet to be bioacustically characterized.