Recibido: 21 de enero de 2021; Aceptado: 2 de junio de 2021
Abstract
Aquatic species are among the least studied groups of snakes in Colombia. This is mainly due to their ecology, which makes some species difficult to survey, resulting mostly in fortuitous encounters. Within this group is the genus Tretanorhinus, for which there are records of two species in Colombia, T. mocquardi and T. taeniatus, along with a possible third one, T. nigroluteus. The presence of T. nigroluteus in Colombia has been problematic, since records are based on misidentifications and incomplete information. Here we extend the distribution of this species to the Pacific region of Colombia and discuss the uncertainty of the previous record. We also compare the morphological variation of the only Colombian specimen currently known with individuals of Central America, finding that this individual differs in having a black dorsal ground color without punctulations and a darkened belly. Nonetheless, we consider this combination of scales and color as previously unknown intraspecific variation. Finally, we report for the first time the presence of dome-shaped cephalic mechanoreceptor sensilla for the genus, quantify its density and size for six cephalic scales, and discuss the possible taxonomic value of these structures.
Keywords:
Aquatic snakes, Mechanoreceptor sensilla, Neotropics, Taxonomy, Variation..Resumen
Las especies acuáticas son uno de los grupos menos estudiados de serpientes en Colombia. Por su ecología, algunas de estas especies son difíciles de muestrear, siendo mayormente encuentros fortuitos. Dentro de este grupo se encuentra el género Tretanorhinus, del cual para Colombia hay registro de dos especies: T. mocquardi y T. taeniatus, y se ha sugerido la presencia de una tercera: T. nigroluteus. La presencia de T. nigroluteus en Colombia ha sido problemática, pues los registros han resultado ser identificaciones erróneas e información incompleta. Aquí actualizamos la distribución de esta especie para la región del Pacífico colombiano, y discutimos la incertidumbre del anterior registro. También comparamos la variación morfológica del único espécimen colombiano con la variación descrita para individuos de Centroamérica, encontrando que este individuo se diferencia por tener coloración dorsal negra sin puntos alternados y un vientre ennegrecido. Sin embargo, por la poca disponibilidad de individuos, consideramos la combinación de escamación y coloración de este espécimen como variación intraespecífica no conocida anteriormente. Finalmente, reportamos por primera vez la presencia de sensilas mecanorreceptoras cefálicas para el género, cuantificamos su densidad y tamaño en seis escamas de la región cefálica y discutimos su posible valor taxonómico.
Palabras clave:
Neotrópico, Sensilas mecanorreceptoras, Serpientes acuáticas, Taxonomía, Variación..Introduction
Despite having a high diversity of species, studies on ecology, evolution, and distribution of most groups of Colombian snakes remain scarce (Lynch, 2012; Lynch et al., 2014). This is particularly true for rare and problematic taxa such as the swamp snakes of the genus Tretanorhinus. Due to their aquatic, nocturnal habits and distribution in regions of difficult access in Colombia, known occurrences are few, making this genus one of the most poorly studied snake groups (Lynch, 2012). This genus is distributed in Central and northwestern South America, including some Caribbean islands. Currently, there are four recognized species within the genus Tretanorhinus: T. variabilisDuméril et al.(1854); T. nigroluteusCope (1861); T. mocquardiBocourt (1891); and T. taeniatusBoulenger (1903). Two of them have records for the Pacific region in Colombia: T. mocquardi and T. taeniatus (Castro-Herrera & Vargas-Salinas, 2008; Vásquez-Restrepo, 2020). However, the taxonomic limits between them are not completely clear (Vásquez-Restrepo, 2020), and hereafter we will treat them as T. mocquardi/T. taeniatus. A third species, T. nigroluteus, has been suggested to be distributed in the Pacific coast of Colombia (Barquero & Arguedas, 2019), based on a review of available information in scientific collection databases. Nevertheless, the occurrence of this species in Colombia is still surrounded with doubts, since older records from Alarcón-Pardo (1978) for the Atlantic coast correspond to a misidentification, and Barquero & Arguedas, 2019 did not provide detailed information about the record they reported, neither reviewed the specimen.
There are numerous records of Tretanorhinus nigroluteus from southern Mexico throughout Central America to Panama, and a disjunct report southward in Colombia (Barquero & Arguedas, 2019). Along with the rare and mysterious ecology of these aquatic snakes inhabiting both fresh and brackish waters, this species is also characterized by having notorious morphological variation in scale counts and coloration patterns (Lee, 1996; Savage, 2002). Five subspecies have been proposed: T. n. nigroluteus, T. n. lateralis, T. n. mertensi, T. n. dichromaticus, and T. n. obscurus, with only the first two being currently recognized (Wilson & Meyer, 1985; Henderson & Hoevers, 1979). Differences among these subspecies are principally based on the color pattern variations and head scutellation. Despite this, variations within these characteristics persist among individuals of the same subspecies (Henderson & Hoevers, 1979).
Historically, taxonomy of snakes has been based on morphological variation in several “macro-traits”, such as bones, scales or hemipenis, whereas interspecific variation in “micro-traits”, such as micro-ornamentations and sensorial organs are less explored. For example, Australian marine snakes have mechanoreceptor cephalic sensilla that vary in number, size and shape between species with different habitats and foraging strategies (Crowe-Riddell et al., 2016; Crowe-Riddell et al., 2019). These structures have also been reported in the anterior cephalic scales of marine elapids and some terrestrial snakes (Jackson, 1977; Jackson & Sharaway, 1980; Young & Wallach, 1998; Crowe-Riddell et al., 2016). Unfortunately, there is limited information on the sensory system of mechanoreception used by mainland aquatic snakes. Henderson & Hoevers (1979) reported the presence of breeding tubercles in the ventral cephalic region of adult males of T. nigroluteus during the breeding season. These breeding tubercles are hypothesized to be enlarged mechanoreceptors that have an important role in stimulating females during courtship or maintaining body contact during mating (Noble, 1937; Henderson & Hoevers, 1979; Crowe-Riddell et al., 2021). Nonetheless, those enlarged mechanoreceptors are season-dependent in T. nigroluteus, whereas normal-sized cephalic sensilla are non-season dependent and seem to have important ecological roles in searching for preys or mates, by detecting movements in specific environmental conditions (Crowe-Riddell et al., 2016; DGC, pers. obs.).
Considering the limited and incomplete information on the presence of Tretanorhinus nigroluteus in Colombia and the lack of information about the morphological variation of the species at the southernmost part of its range, we update its distribution and discuss its variation. Moreover, we report for the first time the presence of cephalic mechanoreceptor sensilla in the genus and discuss the potential taxonomic value of this character.
Materials and methods
We examined the specimens of Tretanorhinus (Appendix I) deposited in the reptile collection of the Instituto de Investigación Alexander von Humboldt (IAvH-R). The identification was based on the original description for T. nigroluteus (Cope, 1861), the revision of the mainland forms of the genus by Dunn (1939), along with more recent studies documenting morphological variations (Barquero & Arguedas 2019; Vásquez-Restrepo, 2020). General measurements of snout-vent length (SVL) and tail length (TaL) were taken using a measuring tape. Sex was determined by subcaudal incision to detect the presence or absence of hemipenes. Scutellation is presented as left/right when not equal for bilateral characters. Finally, multiple photographs of the specimen identified as T. nigroluteus (IAvH-R-2002) were taken with a camera Canon 5D Mark II and piled up with the software Helicon Focus.
To map the distribution of T. nigroluteus, occurrences from Central America were taken from examined specimens cited in the literature (Dunn, 1939; Smith, 1965; Villa, 1969; Henderson & Hoevers, 1979). All records from literature lacked georeference in the original paper, thus, we explored the specimens’ housing museums databases, and georeferenced those without prior coordinate assignment by the museum. We used Google Earth and the described locality with the point method (Wieczorek et al., 2004). We also include data for T. mocquardi/T. taeniatus from Vásquez-Restrepo (2020) and Bioweb Ecuador (access date: January 18, 2021).
To determine the presence of mechanoreceptor sensilla, we first observed the macroscopic protuberant structures in the surface of the head scales in one male of T. nigroluteus and in one male of T. mocquardi using a stereoscope (LEICA S8AP0). We validated the presence of sensilla implementing a modified version of the method used by Jackson & Sharaway (1980), which consists of peeling the outer layer of the epidermis and placing it on a microscopic slide. Posteriorly, the slides with the last layer of epidermis were observed under the stereoscope with the bottom light turned on, which allows a clear contrast between the lighter sensillum and the rest of the scale. This contrast is due to the reduction of the keratin layer just above the sensillum, which is an ultrastructural character of mechanoreceptor organs in squamates (Landmann, 1975; von-Düring & Miller, 1979; Jackson, 1977; Maclean, 1980; Crowe-Riddell et al., 2019). Photographs of these structures were taken using a LEICA MC190 camera integrated into the stereoscope. We measured the number, density, and average size of the sensilla based on the microscopic slides, using ImageJ v1.51. The density was obtained as the number of sensilla.area-1 of the scale (mm2). The counting was performed manually with a counter, and a digital mark was placed on each sensillum registered on the image to avoid miscounting. Finally, the sensillum size in each scale was calculated as the average area (A = πr2) of 10 random sensilla in each scale, calibrated with the stereoscope reference scale.
Results
Distribution
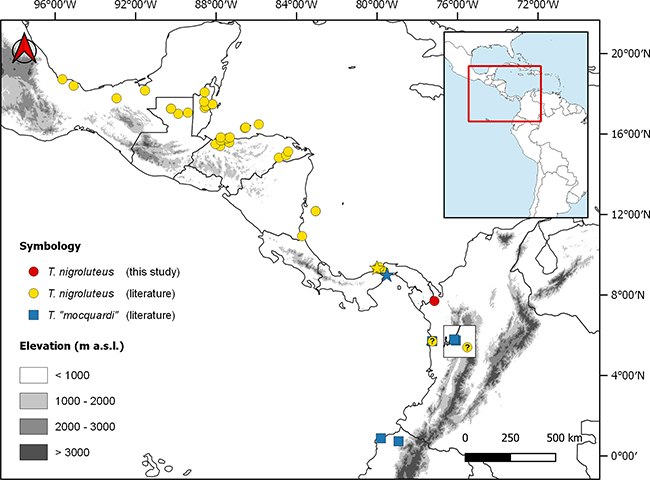
Across its range in Central America, Tretanorhinus nigroluteus seems to be restricted mainly to the Caribbean versant, turning to the Pacific in South America (Figure 1). In the Colombian Chocó, the species is sympatric with its congener T. mocquardi.
Figure 1: Distribution of mainland Tretanorhinus. Stars indicate the type locality of T. nigroluteus (yellow) and T. mocquardi (blue). The inner square shows a zoom to the zone of sympatry of both species in mid-Chocó. T. mocquardi/T. taeniatus are shown under the name “mocquardi”.
New record
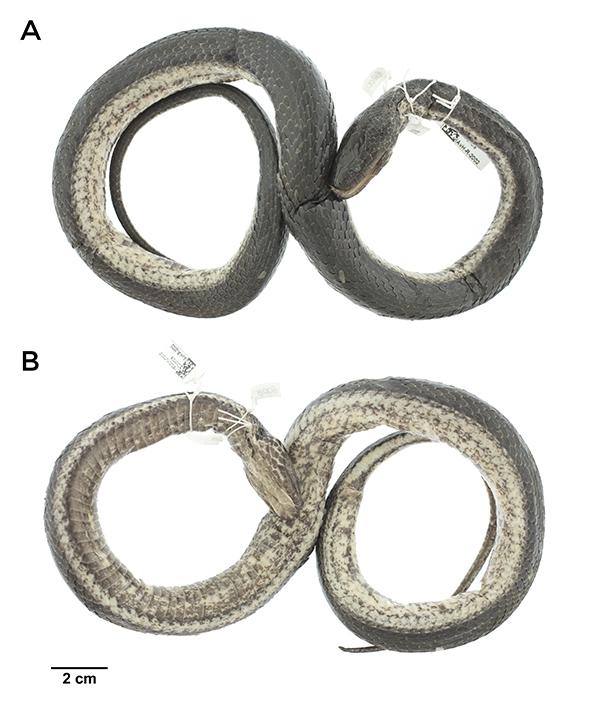
IAvH-R-2002: a male, SVL = 513 mm, TaL = 160 mm. With 21-21-19 dorsal scales rows, strongly keeled; apical pits absent; 2 prefrontals; 2 internasals; 2 loreals per side; 2 preoculars; 2 postoculars; temporals 1+2; supralabials 7/8 (fourth reaching the eye); infralabials 9/10 (1-6 contacting the chin shields); two pairs of chin shields, 138 ventrals; anal plate divided; 66 entire subcaudals. Dorsally with a black homogeneous coloration from the third row of scales to the midline, while the first two rows are cream-colored with some dark blotches (Figure 2A). Ventrally, irregularly dark-spotted, the anterior half more pigmented than the posterior (Figure 2B). The belly also exhibits a diffuse pair of marginal longitudinal dark bands. The tail is light-colored, bordered longitudinally with black, becoming darkened to the tip. Additionally, the holotype of T. nigroluteus (Figure 3) presents a clear longitudinal stripe from the neck extending through the first third of the body, a characteristic absent in the Colombian specimen (Figure 2). This occurrence is about 220 km to the north from the previously reported locality in the vicinity of Nuquí.
Figure 2: Dorsal (A) and ventral (B) views of Tretanorhinus nigroluteus (IAvH-R 2002) from Colombia, Chocó department, Riosucio municipality, vereda Cacarica.
Figure 3: Holotype of Tretanorhinus nigroluteus (USNM 5568) from Aspinwall (now Colón), Panamá (according to Dunn (1939) erroneously given as Greytown, Nicaragua). Photographs from the Smithsonian National Museum of Natural History web site under a CC0 license.
Macroscopic sensilla morphology
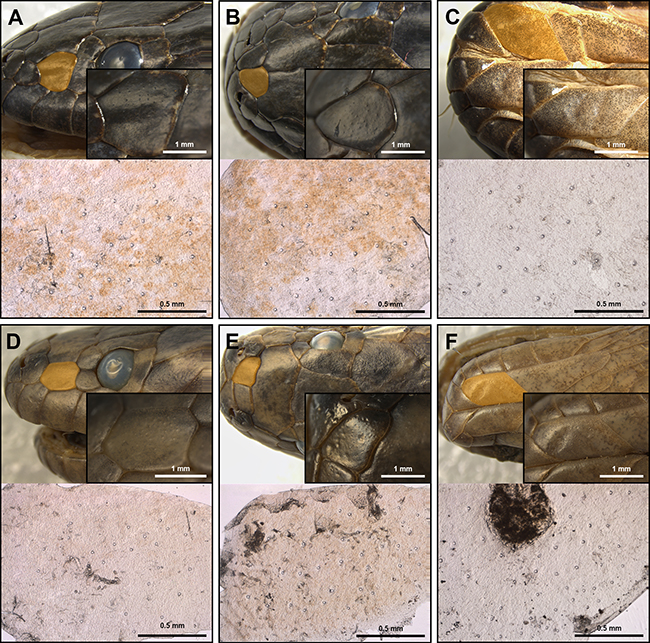
Mechanoreceptor sensilla are observed under the stereoscope as small dome-shaped structures within the cephalic scales of both Tretanorhinus nigroluteus (Figure 4 A-C) and Tretanorhinus mocquardi (Figure 4 D-F). The presence of sensilla was detected in the second and last supralabials, parietal, genial (chin shields), loreal, and internasal scales, being presumably more abundant in the anterior cephalic scales (Table 1). In both species, sensilla are more abundant within the second supralabial, followed by the parietal and genial, compared to the other scales analyzed. As to the density of the sensilla, the second supralabial greatly exceeds the other scales, followed by the internasal and loreal for both species.
Figure 4: Mechanoreceptor sensilla in three cephalic scales of T. nigroluteus (A-C) and T. mocquardi (D-F). Scales are highlighted in yellow. Loreal scale (A, D), internasal (B, E), first chin shield/genial (C, F). Microscopic slides highlighting the sensilla are shown below the cephalic region image.
Table 1: Number, density, and sensillum average size for six cephalic scales of T. nigroluteus and T. moqcuardi.
Discussion
Barquero & Arguedas (2019) reported the presence of T. nigroluteus in Colombia for the first time, after a previous record by Alarcón-Pardo (1978) for the Atlantic coast turned out to be misidentified specimens of Thamnodynastes (ICN 2084-85). In their review, Barquero & Arguedas (2019) used the records available in the databases of several collections, but did not examine nor corroborate the identity of the specimens in which these records are based. Additionally, they just provided a list of museum names along with a map lacking detailed information, and they did not provide voucher numbers nor explicit localities. An issue with data retrieved from online databases is to deal with the taxonomic validation of the records (Maldonado et al., 2015; Marshall & Strine, 2019; Chapman et al., 2020), especially for those supporting new records or range extensions. Unfortunately, it was not possible to access a specimen (UTCH:COLZOOCH-H:0632), which is likely the same referred by Barquero & Arguedas (2019) from the Colombian Chocó. Anyway, we encourage future researchers to be cautious with data from databases, since identifications may be provisional, outdated, and/or have errors. For instance, T. nigroluteus may be confused with Hydromorphus concolor, another aquatic sympatric snake distributed in Central America, then, validated museum data are necessary.
Tretanorhinus nigroluteus seems to be restricted mainly to the Atlantic versant in Central America. However, there are some records to the Pacific in the lowlands of southern Mexico and Nicaragua, in the extremes of Middle America’s mountain ranges (Barquero & Arguedas, 2019). In Colombia, Tretanorhinus are infrequently found, compared with other aquatic snakes such as those of the genus Helicops, which are more frequently sighted and well-represented in collections. T. nigroluteus reaches Colombia through the Darién to the mid-Chocó region, where it seems to be replaced southward by T. moqcuardi/T. taeniatus (Figure 1).
Although data on scale counts for several of the mainland species of Tretanorhinus are broadly incomplete, and variation accounts are based on a limited number of samples (Barquero & Arguedas, 2019; Vásquez-Restrepo, 2020), the morphological traits of the examined specimen agree with T. nigroluteus, compared to other species. Nevertheless, ventral coloration differs substantially from the type (T. n. nigroluteus). This individual also has a particular resemblance in scale counts and partially within its dorsal coloration, to what was once called T. n. obscurus from Nicaragua (Villa, 1969). This resemblance is based on the black homogeneous coloration from the third row of scales to the midline, and the absence of obscure dorsal alternating dark irregular blotches that is characteristic of the other subspecies of T. nigroluteus. Furthermore, as in the populations of Panama and Nicaragua, the specimen reported herein has two loreal scales and differs from specimens in Belize, Honduras, and Guatemala, which have a single one (Dunn, 1939). Thus, here we are considering the specimen IAvH-R 2002 as an intraspecific variation for T. nigroluteus, in absence of any evidence that allows us to assign it to a different entity.
Despite ground coloration variability (olive, grayish, brown, or black), T. nigroluteus and T. moqcuardi/T. taeniatus are readily distinguishable by their dorsal patterns (with or without alternating spots in T. nigroluteus vs. longitudinal lines in T. moqcuardi/T. taeniatus). However, the ventral color pattern has been a subject of interpretation and controversy. Ventral coloration is also quite variable at the intraspecific level, both in color (red, orange, tan, or cream) and pattern. The ventral pattern of T. nigroluteus (T. n. nigroluteus) was partially described by Cope (1861), mentioning just some punctulations to the anterior portion of the body, becoming narrow bands to the edges of the ventral scales, and some dark spots in the subcaudals (Figure 3). Posteriorly, Dunn (1939) mentioned the presence of three longitudinal lines for southern individuals, similar to the ventral pattern observed in T. mocquardi (Vásquez-Restrepo, 2020). Furthermore, Smith (1965) and Villa (1969) suggested additional variation, with venters heavy dark pigmented or with stripe traces. This broad variation leads us to think that there may be hidden diversity under the subspecies names and synonyms, thus a deep and integrative re-examination of the species is necessary.
Our findings on the presence of mechanoreceptor sensilla within the cephalic scales of T. nigroluteus and T. mocquardi are of great interest, yet not unexpected among these aquatic snakes that inhabit turbid brackish waters. Although we did not characterize the sensilla under scanning electron microscopy (SEM) and histological techniques, the external morphology resembling a dome-shape structure, the thinner keratin layer just above the sensillum, along with the greater abundance in the anterior cephalic scales, are in agreement with characteristics of mechanoreceptor organs previously described in other groups of marine and terrestrial snakes (Jackson, 1977; Jackson & Sharawy, 1980; Young & Wallach, 1998; Crowe-Riddell et al., 2016). The distribution of higher number and density of mechanoreceptor organs in the anterior supralabial scales is a pattern reported for the integumentary sensorial organs (ISO) in crocodilians and the sensilla of the Texas rat snake Elaphe obsoleta. It is hypothesized that these organs have an ecological function, providing more sensibility in this region and facilitating the capture of prey items close to the snake’s mouth (Jackson & Sharawy, 1980; Leitch & Catania, 2012). Furthermore, our inferences based on sensillum size are limited by the low number of samples available. For instance, T. nigroluteus seems to have a larger sensillum size compared to T. mocquardi, but this is probably caused by an allometric size effect, as well T. mocquardi seems to have a greater density than T. nigroluteus. Dealing with the allometric size effect may be easily addressed with a size-correction procedure, but a larger number of individuals is needed. Although all evidence suggest that the protuberant round-shaped structures within the cephalic scales of T. nigroluteus and T. mocquardi are mechanoreceptor sensilla, we emphasize that studies under SEM and histological sections are needed for an adequate description of these structures.
Shape, size, prominence, and densities of the sensilla are traits that vary among species of fully aquatic and semi-aquatic snakes and it is hypothesized to be related to differences in ecological aspects (Crowe-Riddell et al., 2016; DGC, pers. obs.). This might suggest that mechanoreceptor sensilla have a potential unexplored taxonomic value for closely related species with ecological differences, for example in prey type, foraging mode or habitat use. Traditional taxonomy based on external morphology is sustained largely in scale counts, which in many cases overlap. As a result, exploring new traits becomes necessary before getting into the concept of cryptic diversity.