Resumen (es):
Resumen (en):
Palabras clave:
Demography, ex situ Conservation, Jaguar, Reproduction, Studbook (en)
https://doi.org/10.21068/c2020.v21n01a06
Demographic analysis for the reproductive management of captive jaguars (Panthera onca) in Colombian zoos
Análisis demográfico para el manejo reproductivo de jaguares (Panthera onca) cautivos en parques zoológicos de Colombia
Santiago Jiménez González
Norbeck Animal Clinic
Silver Spring, United States of America
Haydy Monsalve Redwan
Zoofundación Santa Cruz
San Antonio Del Tequendama, Cundinamarca, Colombia
María Angélica Moreno Duarte
Bioparque Ukumari
San Pereira, Colombia
Claudia Jiménez Escobar
Universidad Nacional de Colombia
Bogotá, Colombia
Recibido: 1 de agosto de 2018
Aprobado: 29 de noviembre de 2019
Abstract
A demographic and reproductive analysis of the population of jaguars (Panthera onca) (n = 38) held in Colombian zoos. Records were analyzed (1970-2010), where paternity and maternity data were completed by DNA’s microsatelite analysis. The SPARKS program was used for the construction of the Regional Studbook for Jaguars and the Population Management 2000 (PM 2000) program for its analysis. The main results obtained were: the census, distribution by sex and age, fertility rate, survival rate, mortality rate, average life expectancy, reproductive value, reproductive projections for 5, 10, 15, 20 and 100 years while preserving the 90% of genetic diversity. The most relevant findings were: the population analyzed in 2010 was decreasing (λ = 0.9876), the generational time (T) was longer for females (T = 18.21 years) than for males (T = 17.46 years), the genetic diversity was on the rise from 1994 to 2002 and remained stable from 2002 to 2009 (GD = 0.78). The genetic analysis allowed to structure the captive population, optimizing the analysis and the reliability of the results, opening the possibility of applying these methods as a conservation model in other threatened species.
Key words. Demography. ex situ Conservation. Jaguar. Reproduction. Studbook.
Resumen
Se caracterizó demográfica y reproductivamente la población cautiva del jaguar (Panthera onca) (n=38) en zoológicos colombianos. Se analizaron registros (1970-2010), en los que se completaron datos de paternidad y maternidad mediante análisis de microsatélites de ADN. Se obtuvieron el censo, la distribución por sexo y edad, las tasas de fertilidad, supervivencia y mortalidad, la expectativa de vida media, el valor reproductivo y proyecciones reproductivas para 5, 10, 15, 20 y 100 años, conservando el 90 % de la diversidad genética. La población analizada en el 2010 estaba decreciendo (λ= 0.9876), el tiempo generacional (T) fue mayor para las hembras (T=18.21 años) que para los machos (T= 17.46 años) y la diversidad genética estuvo en ascenso entre 1994-2002 y se mantuvo estable entre 2002-2009 (GD=0.78). El análisis genético permitió estructurar la población cautiva, optimizando el análisis y la confiabilidad de los resultados, abriendo la posibilidad de aplicar estos métodos como modelo de conservación en otras especies amenazadas.
Palabras clave. Demografía. Conservación ex situ. Jaguar. Reproducción. Studbook.
Introduction
The jaguars of Colombia are classified as a threatened species. They are listed in Appendix I of CITES (https://cites.org), accepted by the Colombian government through Law 17 of 1981. This appendix contains the species of animals and plants in which there is a greater danger of extinction and for which international trade in specimens is generally prohibited, except for certain legal traffic under exceptional conditions. Since 2006, the species has been classified as Near Threatened (NT) in the Red List of the International Union for Conservation of Nature (IUCN) (http://www.iucnredlist.org). The maintenance of a genetically viable captive population of endangered or threatened species has been a primary objective of zoos worldwide since the 1980s (Juffe-Bignoli et al., 2016). Animal preservation programs in zoos seek to establish self-sustaining populations through demographic, genetic and reproductive studies aimed at strengthening captive breeding programs. This is the basis for the implementation of the Species Survival Plans (https://www.aza.org), which seek to have captive populations with high genetic variability, low consanguinity rates and viable populations (Ballou et al., 2010). The Species Survival Plan attempts to maintain as much genetic diversity as possible, setting a reasonable objective of maintaining 90% of that variability over a period of 100 or 200 years, through demographic, genetic and reproductive analyses in a captive population (Lacy, 2013). In this way, populations can be established that serve as an ex situ genetic reservoir for the conservation of species in their natural areas of distribution, contributing positively to in situ conservation. In Colombia there are no studies on the management of captive populations, especially of large carnivores. For this reason we carried out a demographic and reproductive analysis of captive jaguars in Colombia, obtaining kinship information from DNA samples and records, etc. We proposed a population management plan for the species, which provided the necessary tools to establish a sustainable ex situ conservation program for the jaguar in zoos in Colombia.
Materials and methods
Data collection. The construction of the Jaguar Regional Studbook (Panthera onca) (n = 40) for Colombia was carried out using the Single Population Analysis and Record Keeping System (SPARKS) program, developed by the International Species Information System (2005). A Studbook number was assigned for each animal that begins with the prefix T (Temporary), while each animal received a definitive number or code in the International Studbook for the species. Information relating to gender, date of birth, and origin of the parents was listed, which could be from wild animals or from a pair with assigned or unknown temporary numbers. The most relevant events in the life of the specimen were collected, such as birth, transfers, loans, and death with their respective location and date of occurrence of the events.
All the zoos in the country, belonging to ACOPAZOA (Colombian Association of Zoological Parks and Aquariums), were contacted. Information was collected from the zoo records for the last 30 years through a survey where all the general and medical information of the specimens was recorded in the seven institutions belonging to the association. The records of the Cali Zoo were not included in the analysis, since the information was not made available.
Animals. In order to construct the population pedigree blood samples were obtained from captive jaguars (n = 15) under general anesthesia in three zoological parks: Santacruz Zoo (n = 8) in San Antonio del Tequendama, Cundinamarca (6 ° 13 ' 23.93 "N, 75 ° 34'48.81" W), Matecaña Zoo (n = 6) in Pereira, Risaralda (4 ° 49'0.30 "N, 75 ° 44'15.04" W), Piscilago Zoo (n = 1) in Nilo, Cundinamarca (11 ° 0'39.84 "N, 74 ° 47'53.69" W). Hair follicles were also obtained from the jaguars of the Santafé Zoo (n = 5) in Medellín, Antioquia (6 ° 13'23.93 "N, 75 ° 34'48.81" W). For the construction of the pedigree, the information was included compiled in the Regional Jaguar Studbook for Colombia of the animals that were not sampled at the Piscilago Zoo (n = 2), the Barranquilla Zoo (n = 3), Bioparque los Ocarros (n = 2), Cali Zoo (n = 2) and Jaime Duque Park Zoo (n = 3).
Anesthesia. After a fasting period of 24 hours, an anesthetic protocol was performed with the use of a xylazine-ketamine combination. Xylazine, a sedative that is an alpha 2 adrenergic agonist, was given as an initial injection. Five minutes after application and once signs of sedation were observed, a second injection was applied with a fixed dissociative anesthetic derived from cyclohexylamines (ketamine). Drug administration was performed using remote drug injection devices (DIDD). The site of the impact of the dart was located in the muscular masses of the posterior and anterior limbs, the former being preferred, the objective being for the dart or injection to be applied in the most caudal portion of the muscle mass and avoiding a possible impact on the femoral bone or sciatic nerve. The doses of drugs administered were calculated according to the weight reports recorded in the medical records and the doses reported for jaguars by Jiménez & Moreno (2004) and Jiménez et al. (2017a).
Sampling. Blood samples were obtained by puncturing the femoral vein or the saphenous vein after disinfecting the area with alcohol. The samples were collected in vials with EDTA (10 ml) and kept at 4° C, until arriving at the laboratory (Jiménez et al., 2017a).
DNA analysis
Primers. The selected primers were designed for domestic cats (Menotti-Raymond & O'Brien, 1995). The criteria for the selection of microsatellites were based on the fact that all of them should be on different chromosomes and that their use was reported in jaguars (Eizirik et al., 2001, Ruiz-García et al., 2006). The selected microsatellites were fluoromarked with fluorochromes that did not match each other, depending on the selected microsatellite. Only the forward sequence of the first was fluorinated (Jiménez et al., 2017a, 2017b).
DNA extraction and quantification. DNA extraction was performed from leukocytes isolated from blood samples, using the salting out technique. The DNA concentration was quantified by spectrophotometry at 260 nm. The temperature for each microsatellite was calculated by means of the thermodynamic formula, which indicated that the average banding temperature necessary to perform the base pairing was 55.5° C. For the hair samples, the Qiagen Dneasy Blood and tissue extraction kit was used, using the instructions (Jiménez et al., 2017a; 2017b).
PCR. PCR reactions for all markers were brought to a total volume of 20 µL. These consisted of 1.5 mM MgCl2, 1.25mM for each of the dNTPs (dATP, dGTP, dCTP and dTTP) plus 0.4 units of Tth DNA polymerase (Thermus thermophilus) and 10 pmol of each primer, 0.5 mg.ml-1 of BSA as well as 4µl of genomic DNA (10 ng). The samples were amplified under the following conditions: initial denaturation at 95⁰ C for 5 min; 35 cycles of denaturation at 95⁰ C for 1 minute; banding temperature corresponding to each marker for 2 min; extension at 72⁰ C for 2 min and subsequently 1 final extension cycle at 72⁰ C for 5 min, using a Multigene Gradient PCR thermal cycler (Labnet, International; Jiménez et al., 2017a; 2017b).
Agarose. After the PCR procedures were performed, the amplification products were evaluated in 1.5% agarose gels, stained with SYRB Green (Invitrogen, USA). Once the amplification of the products was verified by direct visualization, capillary electrophoresis was then applied (Jiménez et al., 2017a; 2017b).
Capillary electrophoresis. Capillary electrophoresis was performed on an ABI PRISM® 310 Genetic Analyzer. The results of capillary electrophoresis and the appointment of alleles were determined by direct visualization of the chromatograms in the Softgenetics Gene Marker Version 1.97 program. All samples were amplified and genotyped at least 2 times, to minimize problems of non-assignment (drop out; Jiménez et al., 2017a, 2017b).
DNA Statistical Analysis
To assign paternity and maternity and for the construction of the pedigree of the population, the Cervus version 3.0.3 and Kinship 1.2 program was used. For the final construction of the pedigree, the following variables were taken into account: information obtained from the Studbook (sex, date of birth, previous knowledge of fathers and mothers), natural logarithm values of likelihood relations (LOD), assigned by Cervus with 80% flexible and 95% strict confidence intervals (Jiménez et al., 2017a, 2017b).
Likewise, the relationship and kinship probabilities assigned by Kinship for H1 (Rp = 0.5 and Rm = 0.5) vs. H0 (Rp = 0 and Rm = 0; α = 0.05, 0.01 and 0.001) were considered.
The variables define the likelihood of a jaguar pair sharing an allele by direct descent from their father or mother. For example, if the hypothetical relationship is two complete siblings, both r values would be 0.5. After the hypothesis is given, kinship uses the r values, allelic frequencies and genotypes of two individuals under consideration to calculate the likelihood that this combination of genotypes could have been produced as specified (Goodnight, 2004; Jiménez et al. , 2017a, 2017b).
Analysis of records data. The information collected was entered into the Sparks program where a preliminary analysis was made. It was subsequently exported to the Population Managment 2000 program, in order to carry out the demographic analysis in which the census and distribution by gender and age were initially evaluated (Pollak et al., 2005). Additionally, the following parameters were established (Lacy & Ballou, 2002, Pollak et al., 2005) as listed below:
Age distribution was calculated as the average number of animals in each age class that would be expected to be found if the proportions of the life table had been constant over time. These are defined by the survival rate for each age that is presented in the life table by sex. The distribution by sex and by age is determined with the current numbers, and the distribution of expected ages with the stable numbers (Caughley, 1977).
Growth (λ) was calculated from the growth rate per generation R0 (Rates of change in population size per generation equivalent to the average number of juveniles of the same sex that a parent produces throughout their life) and the average duration of a generation G.
Nt = erxt x N0
r=Ln (Nt/N0)/t
r= Ln (Ro)/G
λ = er
The proportion of annual population growth was compared with that of the previous year and was denoted by lambda (λ) and/or by the demographic reduction r = (1- λ) for a population under stable conditions.
Age-specific mortality rate (Qx) was calculated as the proportion of individuals in the age class X which dies before reaching the age class X + 1. Qx should be calculated from the mortality data. It is calculated by dividing the number of animals that die during their passage by an age class (x) by the number of animals that were alive at the beginning of the same age class and is inversely proportional to the survival rate. The trend lines were obtained for each rate that show the estimates for the model population after the data was smoothed, eliminating the extreme peaks and replacing each point with the average of the predecessor value and the following number. This procedure can be done multiple times and according to the number of times it is denoted 1x, 2x ... etc.
Age-specific survival rate (Px) was calculated as the proportion of individuals that arrive alive from the beginning of the age class (X) to the next age class (X + 1).
Age-specific survivorship (Lx) was calculated from the proportion of individuals that survived from birth to the beginning of age class X. For the first age class (x = 0, between 0 and 1 year of age). Survival at age 0 is defined as Lx = Lo = 1.
Example: Of the females of class 0, a proportion Po survived until the beginning of class 1, thus I1 = Po. Of the females in age class 1 a proportion P1 survived towards age class 2, thus L2 = L1 x P1 (or L2 = P0 x P1), in general terms;
For all age classes except the first (x = 1 to 5)
Lx = P0 x P1… x Px -1
= L-1 x Px-1
Age-specific fertility rate (Mx) is the average number of offspring of the same sex of the parent, born of an individual in the age class X. Example: it can be calculated as the number of females born in the age class X divided by the number of females at the beginning of the age class X.
Median life expectancy (Ex) was calculated from the average number of years of life remaining that an individual in an age class (x) can reach.
Reproductive value (Vx) measures an expectation. In this case, the expected number of descendants in the current year and in future years for an age class animal (x) was calculated (Pollak et al., 2005).
Once this was obtained, the reproductive projections of the population were carried out, and the population goals were established in order to maintain 90% of the genetic diversity over a period of 100 years (Lacy et al., 2002). The variables that were considered for reaching this population goal were as follow: generational time (T); population growth rate (λ); current population size (N); current effective population number (Ne); Ne / N ratio; current genetic diversity (GD); maximum population allowed; new founders per event of addition; year of introduction of new founders; year of cessation of introduction of new founders; and the years between events of addition (of new founders).
Generational time (T) was considered as the time elapsed between the first reproductive event of one generation and the next or the mean age at which a parent produces offspring (Li et al., 2009).
Finally, the pairing selection was made. The criterion used for pairing selection was the value of the mean of kinship (Mean Kinship, MK), which was defined as the average of the coefficients of relationship that exist between an individual (including that individual) and the living population of animals born in captivity. The convenience of zoological institutions and the geographical location of the specimens were also used as criteria. The result that was obtained for the modeling of the offspring was given by the inbreeding coefficient (F) obtained, looking for the offspring in the virtual generation with F values equal to zero. Taking into account the information in the Studbook records, the paternity and maternity allowances for the Cervus program and the kinship relationships obtained by Kinship, the construction of the pedigree for the population of captive jaguars in zoological parks in Colombia was carried out.
Results
When constructing the pedigree, an association was found between the animals of the Matecaña and Santafé zoos. A high genetic relationship between the jaguars of the Santacruz Zoo was determined and connections were also found in the Studbook records between this zoo and the zoos of Piscilago, Barranquilla and Jaime Duque.
In summary, four maternity units (T15, T25, and T19 twice) and five paternities (T17, T26, T3 and T16 twice) were assigned through the Cervus program. Four paternities (p <0.001) (T17, T18 and T5 twice) and one maternity (T6) were assigned both by Cervus and Kinship. Likewise, four maternity units (p <0.01) were assigned by Kinship for two individuals (T15 twice and T6 twice). A significant relationship (p <0.05) was found between the jaguar T17 and the jaguars T23, T19 and T24. Subsequently, the Jaguar Regional Studbook for Colombia was updated including the paternity and maternity units that were assigned by Cervus that presented an exact comparison and relations by Kinship (p <0.001).
General characteristics of the population
For 2010 the population of live jaguars was 25 specimens consisting of 44% (n = 11) males and 56% (n = 14) females. Of the population 80% (n = 20) corresponded to specimens of spotted phenotype (Figure 1) and 20% (n = 5) of the individuals corresponded to the melanistic phenotype (all females; Figure 2). At the reproductive level the population was composed of intact females (100%, n = 14), intact males (81.8%, n = 9) and vasectomized males (18.2%, n = 2). The ratio of males and females is, therefore, on the order of three females for every two intact males (3: 2). Of the population 76% (n = 19) are between 9 and 16 years old. Only one male and two females were younger than 9 years (12%) and two females and one male were older than 16 years (12%).
From the total census and the comparative census of the population of jaguars that have come from the wild, those born in captivity were calculated. A notable differential population increase was found in male jaguars as compared to females between 1990 and 2000.

Figure 1.Spotted jaguar adult male. Santa Fe Zoo, Medellín Antioquia (Foto: Santiago Jiménez).

Figure 2. Melanic jaguar adult female. Santacruz Zoological Foundation San Antonio del Tequendama, Colombia (Foto: Santiago Jiménez).
From the year 2000, there was a decrease in the total population numbers, mainly in the male group (Figure 3). The population increase observed was mainly due to the birth of captive animals, since the number of individuals from the wild remained constant. For the population of 25 specimens registered in 2010, there were 4 wild-born jaguars and 21 born in captivity (Figure 4). The population structure is of the rhomboid type, which occurs in populations without young individuals, a large number of individuals in middle age and a few in advanced age (Figure 5).
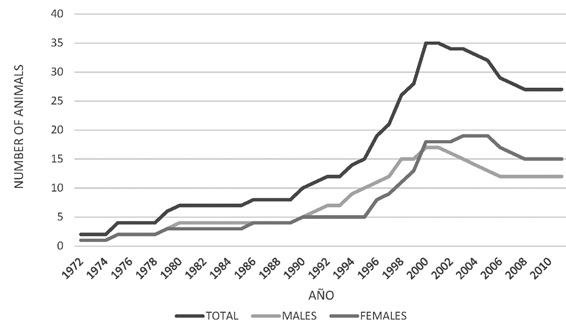
Figure 3. Census of captive jaguars (Panthera onca) (Males, females and total) in captivity in Colombia 1970 y 2010.
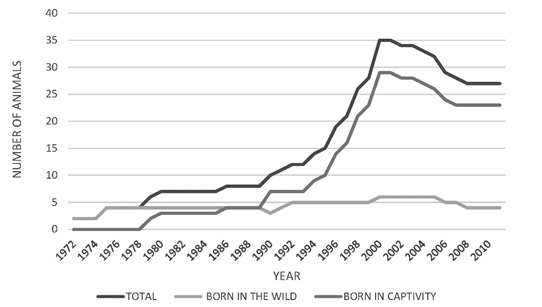
Figure 4. Census of captive jaguars (Panthera onca) (captive born vs born in the wild) in Colombia 1970 y 2010.
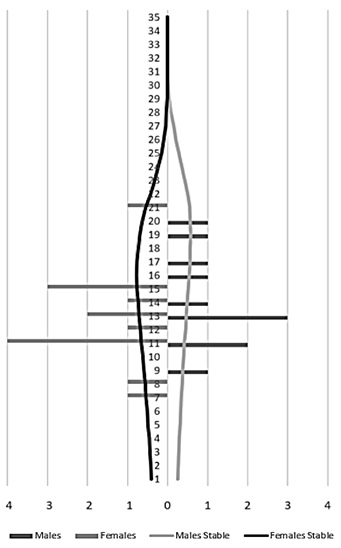
Figure 5. Age class distribution (Panthera onca) Males on the left (n=11) females on the right (n=14).
Age-specific fertility rate (Mx). In males the first reproductive event where fertilization occurred was at 2 years of age and the last at 22 years. The moment of maximum reproduction occurred between the ages of 16 and 22. For females the first fertile reproductive event also occurred at 2 years, the last at 25 years, and the moment of maximum reproduction occurred between 17 and 25 years (Figure 6).
Age-specific survival rate (Px). Males from 16 years of age showed a differential survival probability of 1. By age 22, only 50% (P22 = 0.5) of the animals will pass to the next age class and after 31 years no survivors are expected (P31 = 0). As for females, it was found that after 14 years of age the probability of survival was less than 1 and continues decreasing. Around 24 years, only 50% (P24 = 0.5) of the animals will move on to the next age class and after 32 years, no survivors are expected (P32 = 0; Figure 7).
Age-specific survival (Lx). In males the survival rate decreased after 7 years and none survived after 32 years (L32 = 0). For females the decrease in survival rate occurred after age 8, and none survived after age 33 (L33 = 0; Figure 8).
Age-specific mortality rate (Qx). A 5% mortality rate was observed in males after 7 years (Q7 = 0.05), 35% between 21 and 24 (Q21-24 = 0, 35), 50% between 25 and 27 (Q21-24 = 0.5), and 100% after 30 years (Q30 = 1). As for females, the mortality rate was 3% from 8 years (Q8 = 0.03), 20% at 16 years (Q16 = 0.2), 30% at 20 years (Q20 = 0.3), 38% between 22 and 24 years (Q22-24 = 0.38), 50% between 24 and 26 (Q24-26 = 0.5) and 100% after 31 years (Q31 = 1).
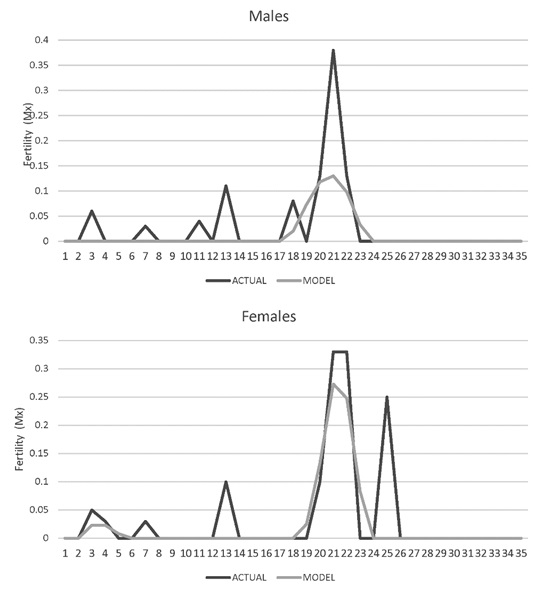
Figure 6. Fertility rate age specific (Mx) for jaguars (Panthera onca) for males and female jaguars (Panthera onca) in captivity in Colombian Zoos.
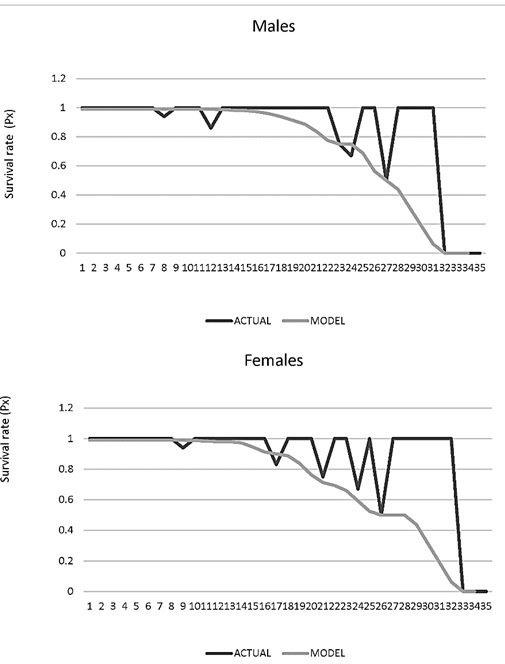
Figure 7. Survival rate age specific (Px) for males and female jaguars (Panthera onca) in captivity in Colombian Zoos.
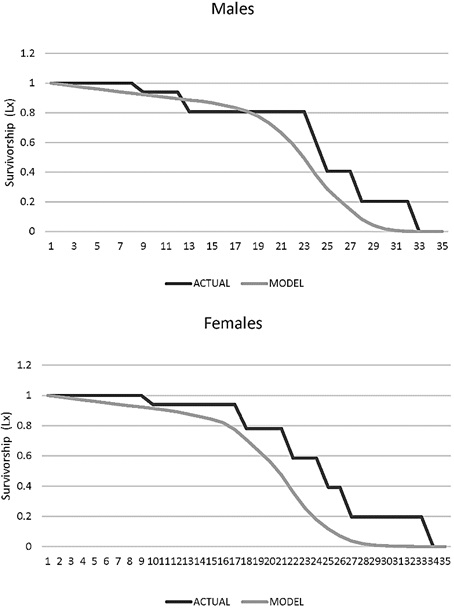
Figure 8. Survivorship age specific (Lx) for males and female jaguars (Panthera onca) in captivity in Colombian Zoos.
Average life expectancy. The average life expectancy was calculated for both males and females. The average life expectancy in females was lower than for males in the first 24 years of life. At 10 years the remaining life expectancy for females is 10 years while for males it is close to 12 years; after 20 years the life expectancy remaining for both males and females is less than 5 years (Figure 9).
Reproductive value (Vx). It was found that the reproductive value of females tends to be greater between 15 and 23 years. That is, the reproduction of jaguars after 23 years of life would hardly be carried out (Figure 9).
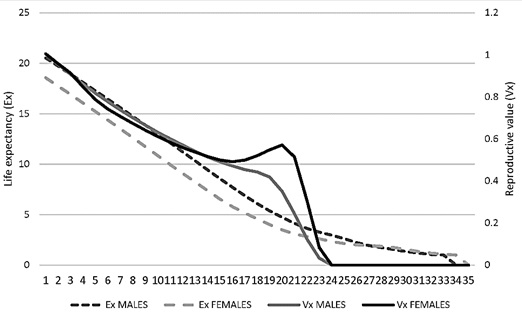
Figure 9. Reproductive value (Vx) and life expectancy (Ex) for males and female jaguars (Panthera onca) in captivity in Colombian Zoos.
Reproductive projections for males and females. The current population represents 99% of that of the previous year (λ = 0.9876). The generational time (T) was longer for females (T = 18.21 years) than for males (T = 17.46 years). For the population model, with no reproductive management, the data shows that there would be a decrease in the population of males by 7% (λ = 0.9313) and females by 3.3% (λ = 0.9667), as well as an increase in generational time, both for males (T = 20.64) and for females (T = 21.92). Including these variables and taking into account the current number of males and females, we estimated that the projected number of males and females at twenty years (N20) would be 2.7 males and 7.1 females (Table 1). Using this data we calculated a graph of total reproductive projections including both males and females, without a reproductive management plan. Population decline to less than 10 individuals was observed taking into account the mortality rate. However, a projection of the population growth at 20 years using reproductive management was carried out as well, which increased the current number from 25 specimens to 40 adjusting capacity in the zoos of Colombia and assuming that the sex ratio of the offspring at birth was 50%. From this information, the births that were needed to reach the size of the target population, the level of population growth (λ) and the number of pairs needed were determined. These results determined that in order to reach the expected population number with reproductive management for 5 years, a population growth of 9.8% per year (λ = 1.098), with 3.3 births per year and 6.6 pairs would be needed to reach this goal. With reproductive management for 10 years, a population growth of 4.8% per year (λ = 1.048) would be required, with 2.1 births per year and 4.2 pairs of jaguars would be needed to reach the population goal. With reproductive management for 15 years, a population growth of 3.1% per year (λ = 1.031) would be needed and 1.7 births per year and 3.4 jaguar pairs would be needed to reach the population goal. With reproductive management for 20 years, a population growth of 2.3% per year (λ = 1.023) would be needed with 1.5 births per year (in other words, 3 births in 2 years) and 3 pairs of jaguars would be needed to reach the population goal. (Table 2)
Table 1. Demographic characteristics for the captive jaguar population in 2010 (Panthera onca) in Colombian zoos.
Table 2. Projections for the captive population of jaguars (Panthera onca) with reproductive management for 5, 10, 15 y 20 years.
Population Goals. The goal for jaguars in Colombia is to maintain 90% genetic diversity of the wild jaguar population over a period of 100 years. Population goals were obtained without involving any type of reproductive management (Figure 10). There was a decrease in the constant population numbers and loss of genetic diversity that drops from 75% between the first 10 and 20 years. With reproductive management simply increasing the population from 25 to 37 specimens and by introducing a founding animal every year, genetic diversity would increase from 78% to 90 % in the time between 40 and 50 years of management, and generational time would decrease from 21 years to 12 years. The selection criteria for pairing used the average kinship value and the geographic location.
A population goal was proposed to conserve 90% of the genetic diversity for 100 years (Figure 11), taking into account the following variables: generation time (T = 12); population growth rate (λ = 1.02); current population size (N = 25); current effective population number (Ne = 6.5); Ne / N ratio (0.26); current genetic diversity (GD = 0.78); maximum population allowed (n = 40); population required to reach goals (n = 37); and the equivalent of founder genome (EGF) recruited by each new founder (FGE = 0.4; Table 3).
Table 3. Population goals for captive jaguars (Panthera onca) in Colombian zoos, with and without reproductive management for 100 years.
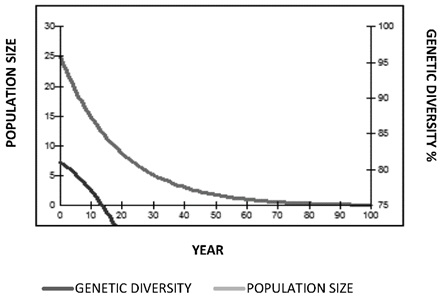
Figure 10. Population goals projections for 100 years (Panthera onca) without reproductive management.
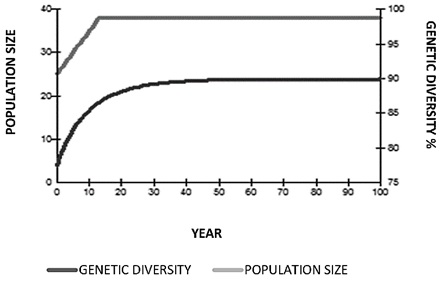
Figure 11. Population goals projections for 100 years (Panthera onca) with reproductive management.
Matching selection. The male and female specimens were organized according to their MK kinship value. It included the males and females alive in 2010 with 100% of the known pedigree that when mating with each other would have a progeny with an F = 0.
Discussion
During the research, limitations were found regarding the type of records handled in the different zoos. The records date from the 80s and 90s and are not uniform for the type of information collected. Instead the records depended on the criteria of each professional in charge of the collections, leaving gaps with each administrative change, and they did not obey a general consensus among all the zoos. For this reason the loss of information is high for the movement of animals, deaths and births. However, the situation changed in 2000, when all zoos unified their types of records. From this date more complete information about each animal is found. Other limitations during the characterization of the population of captive jaguars in Colombia are similar to those found in the organization of the jaguar studbook in European zoos (Drury et al., 2005), with the difference that in Europe these data have been recorded for nearly a century, while in Colombia the development of this activity is only 35 years old. Similar difficulties arose in the elaboration of the jaguar studbook in Mexico (Rueda-Zozaya et al., 2016).
In 2010 the population of Colombian jaguars was composed mainly of adults over 10 years. This indicated that in the future there would be a population decline in captive jaguars in Colombia, if there was no generational relief, either by captive breeding providing a population base of young individuals or by the addition to the population of wild-origin specimens. Wild jaguars have their origin from illegal animal traffic, confiscated by environmental authorities, and, after a rigorous evaluation, it is concluded that they would not survive in an eventual release (Zarrate-Charry et al., 2009).
This research was conducted as a model that could be implemented for any captive species. By having a variable population in genetic terms, the conservation level impact is extraordinary, because any wild population on the verge of extinction and with poor genetic variability would benefit from receiving new genetic material from a healthy captive population. As for the release of individuals, the impact would be much less for the revitalization of a threatened population. The contribution would be significant if a structured population of individuals is released in which reproduction has already occurred and viable offspring have been produced, or by introducing gametes or embryos from the captive population to the wild population, in order for young to be born in freedom to be educated by their mother naturally, increasing the chances of survival (Jiménez et al., 2017a, 2017b; Jiménez, 2017).
The male: female ratio of 2:3 indicates that it is necessary to increase the number of uncastrated males to stabilize the gender ratio, considering that the male: female ratio should be 1: 1 (Drury et al., 2005). This ratio of 1:1 would ensure that the possible male and female founders have the same probability of transmitting their gametes to contribute to an increase of genetic variability of the population, in contrast to how it occurs in domestic animals, in which the ratio can often be one male for several females. In this case only the alleles of that male would be represented in the population, increasing the risks of inbreeding.
Taking into account that melanism is transmitted by dominance and that the spotted expression is expressed by recessive homozygosis, it can be inferred, according to the Mendelian segregation law, that finding that 87% of captive jaguars in Colombia are spotted, there is an excess of recessive homozygous and a deficit of heterozygous and dominant homozygous for the phenotypic expression of melanism (Eizirik et al., 2003).
Evidence was found that jaguars effectively reproduced in captivity between the years 1990 and 2000 and the number of specimens increased from 10 to about 35. This increase in population was mainly due to captive breeding rather than to the entry of animals from wildlife. As of the year 2000, a population decline began, mainly due to the natural incidence of the mortality rate, the interruption of controlled mating and the low increase of the population due to new founding animals.
If it is considered that the reproductive life of Panthera onca begins at 2 years and ends near 25 years, in the captive population the initial age would coincide with the age at which the wild subadults move away from their mother to lead an independent life and when these are considered reproductively suitable. The range of greater specific fertility for the age found for males was between 16 and 22 years and for females between 17 and 25 years suggests that it is a reflection of the moments of controlled reproduction according to managed conditions of the population in each zoo. At present, since there are few animals between those ages, it would be necessary to use the youngest specimens in the processes of controlled or assisted reproduction.
The growth, survival, mortality and life expectancy rates found depend on different factors related both to the nature of the species and to human intervention, represented by permanent care, constant food administration and medical care that is both preventive and curative. Taking these considerations into account and ruling out idiopathic pathologies or accidents, we found that the life expectancy of captive jaguars in Colombia is similar to the 25-year life expectancy reported for jaguars in European zoos (Drury et al., 2005).
In general, the Species Survival Plans try to maintain as much genetic diversity as possible from captive species (Hampson & Schwitzer, 2016) without confiscated animals, which must be progressively reduced with population education programs. Due to space limitations in Colombia's zoos a reasonable objective has been set to maintain 90% of this variability for jaguars for a period of 100 years (Lacy, 2013). In other felids, such as Siberian tigers (Panthera tigris altaica), a time of 200 years has been set. The population analyses in captivity of the Siberian tiger indicate that they have a generational period of seven years, while for captive jaguars this is close to 20 years, due to the current management conditions. But the generational period for jaguars could diminish much more when changing the strategies of reproduction with knowledge of the parental lines of the animals in captivity. The number of founders in the Siberian tiger plan was 34, while the number of founder jaguars in Colombia was 7. As the initial number of founders is so low, 12 to 15 new founders would be needed to preserve the genetic diversity of 90 % for 100 years, while in the case of Siberian tigers another 10 or 12 unrelated founders are needed, so that a population of 175 tigers retain 90% of their genetic diversity over the next 200 years (Seidensticker & Lumpkin, 1996).
To achieve this objective it is necessary to improve the registration databases and to keep the Studbook updated (Flesness, 2003). Given that the population of captive jaguars in Colombia is decreasing (λ = 0.9876), it is essential to implement a reproductive management plan such as the one proposed in this study, to stabilize the population and to avoid losing the gene pool. Thus, it is necessary to promote controlled breeding and the entry of new founders, especially males, to restore the balance of sexes, in order to maintain a pyramid-shaped population structure.
It is also important to reduce the generational time. This can be done by including young individuals in the breeding programs. When doing this, the maximum fertility rate will be at an earlier age, which will be reflected in an increase in the years of reproductive life of each specimen. This program must include an assessment of kinship to maximize genetic diversity. The pairings must look for minimum consanguinity, which is achieved if the pedigrees of each pair are known and the MK and F values are established (Leus et al., 2011).
Camelo (2014) published a Studbook based only on records, different from the studbook presented in this study. That author included the new data from between the years 2010 and 2014 and data from the Cali Zoo. This report does not know the parental and maternal origin of most of the data. The lack of parental information invalidates the results of any reproductive analysis. It is important that the kinship studies continue and be analyzed periodically, in order to assess the status of the captive population and the progress or regression in maintaining the genetic diversity of the population.
Our study resolved part of these questions. The parental origin of eight specimens located in the zoo of Matecaña and Santa Fe (T11, T21, T16, T18, T23, T24, T40 and T9) was elucidated providing a more robust population structure for demographic analysis. Before carrying out the study, the origin of the pedigree of the population of jaguars was known in 28.9% of the cases (n = 11), and after the construction of the population pedigree 55.3% (n = 21) were known. This optimizes the information by 98% and shows the advantages of using microsatellites as a complement in the update of the studbook. From this it was possible to obtain an accurate record of the origin of live specimens in the population, which can be used in current and future population management programs, involving controlled crossings to avoid consanguinity and thus keep the population variable in genetic terms as a research model (Jiménez et al., 2017a, 2017b). In this way this study is a useful tool for zoos to develop their plans for reproduction.
It is also necessary to program matings with prior knowledge of the reproductive physiology of the species. This makes it necessary to carry out research focused on characterizing the jaguar's estrous cycle in tropical conditions through endocrine analysis and on the analysis of gametes, both male and female (Jiménez et al., 2017b; Jiménez, 2017). To conclude, this research has demonstrated the importance of integrating demography, genetics and reproduction as solid pillars in the ex situ conservation of wild felids in Colombia.
Referencias
Ballou, J.D. & Foose, T.J. (1996). Wild Mammals in Captivity. Principles & Techniques for Zoo Management. Chicago: USA: University of Chicago Press. 219 pp.
Camelo, V. (2014). Studbook Nacional de Jaguar (Panthera onca). Bogotá D.C.: Acopazoa. 34 pp.
Caughley, G. (1977). Analysis of vertebrate populations. Chichester, United Kingdom: New York: John Wiley and Sons. 234 pp
Drury, H., Hill S. & Saccheri I. (2005). Investigation into inbreeding en European Zoo Population of jaguars (Panthera onca). Zoo Research News. BIAZA Research Newsletter, 6 (4).
Eizirik, E., Yuhki, N., Johnson, W.E., Menotti-Raymond, M., Hannah, S.S. & O'Brien, S.J. (2003). Molecular genetics and evolution of melanism in the cat family. Current Biology, 13(5), 448-453.
Flesness, N. R. (2003). International Species Information System (ISIS): Over 25 years of compiling global animal data to facilitate collection and population management. International Zoo Yearbook, 38, 53-61.
Goodnight, K.F. (2004). Kinship 1.2. User Manual.
Hampson, M.C. & Schwitzer, C. (2016). Effects of Hand-Rearing on Reproductive Success in Captive Large Cats Panthera tigris altaica, Uncia uncia, Acinonyx jubatus and Neofelis nebulosa. PLoS One, 11(5).
Henkel, J.R., Jones, K.L., Hereford, S.G., Savoie, M.L., Leibo, S.P. & Howard, J.J. (2012). Integrating microsatellite and pedigree analyses to facilitate the captive management of the endangered Mississippi sandhill crane (Grus canadensis pulla). Zoo Biology, 31(3).
International species information system (ISIS). (2005). User manual for SPARKS single population analysis and records keeping system.
Jiménez, S. & Moreno. W. (2004). Manejo y anestesia en cinco especies de félidos en cautiverio en parques zoológicos de Colombia. Revista UDCA, Actualidad y Divulgación Científica, 6, 73–83.
Jiménez, S. (2017). Caracterización demográfica, genética y reproductiva de jaguares cautivos (Panthera onca) en cautiverio en parques zoológicos de Colombia. (Trabajo de grado). Bogotá D. C.: Universidad Nacional de Colombia, Facultad de medicina veterinaria y zootecnia, Departamento de salud animal. 129 pp.
Jiménez, S., Ruiz-García, M., Maldonado, J., Jiménez, L., Sánchez, A., Wilbert, T., . . . Jiménez, C. (2017a). Genetic Characterization of Jaguars (Panthera onca) in Captivity in Zoological Parks of Colombia. En ShrivastavA.B & Singh KP (Eds.). Big Cats. 73-91 pp. Rijeka Croacia. Intech.
Jiménez, S., Howard, J. G., Brown, J., Grajales, H., Pinzón, J., Monsalve, H., . . . Jimenez Escobar, C. (2017b). Reproductive analysis of male and female captive jaguars (Panthera onca) in a Colombian zoological park. Theriogenology, 89, 192-200.
Juffe-Bignoli, D., Brooks, T. M., Butchart, S. H. M., Jenkins, R. B., Boe, K., Hoffmann, M., . . . Kingston, N. (2016). Assessing the Cost of Global Biodiversity and Conservation Knowledge. PLoS One, 11(8), e0160640.
Lacy, R. C. (2013). Achieving True Sustainability of Zoo Populations. Zoo Biology, 32(1), 19-26.
Leus, K., Traylor-Holzer, K. & Lacy, R.C. (2011). Genetic and demographic population management in zoos and aquariums: recent developments, future challenges and opportunities for scientific research. International Zoo Yearbook, 45(1), 213-225.
Li, M. H., Strandén, I. & Kantanen, J. (2009). Genetic diversity and pedigree analysis of the Finnsheep breed. Journal of animal science, 87(5), 1598-605.
Lockyear, K.M., Waddell, W.T., Goodrowe, K.L. & MacDonald, S. E. (2009). Retrospective investigation of captive red wolf reproductive success in relation to age and inbreeding. Zoo Biol, 28(3), 214-229.
Menotti-Raymond, M. & O'Brien, S.J. (1995). Evolutionary conservation of ten microsatellite loci in four species of Felidae. Journal of Heredity, 86(4), 319-22.
Pollak, J. P., Lacy R. C. & Ballou J. D. (2005). Population Management 2000, version 1.213. Brookfield, IL.: Chicago Zoological Society.
Seidensticker, L. & Lumpkin, S. (1992). Felinos. Barcelona: Plaza y Janes. Colección Materia viva. 240 pp.
Rueda-Zozaya, P., Mendoza-Martínez, G. D., Martínez-Gómez, D., Monroy-Vilchis, O., Godoy, J. A., Sunny, A., . . . Herrera-Haro, J. (2016). Genetic variability and structure of jaguar (Panthera onca) in Mexican zoos. Genetica, 144(1), 59-69.
Ruiz-García, M., Payán, E., Murillo, A., Álvarez, D. (2006). DNA Microsatellite characterization of the Jaguar (Panthera onca) in Colombia. Genes and Genetics Systems, 81, 115-127.
Zarrate-Charry, D., Laverde Trujillo, L., Balaguera-Reina, S., González-Maya, J. & Trujillo, F. (2010). Rescate y manejo de fauna silvestre ex situ en Colombia: Estudio de caso de un jaguar (Panthera onca) en la Orinoquía Colombiana.CES Medicina Veterinaria y Zootecnia, 4(1), 81-89.
Referencias
Ballou, J.D. & Foose, T.J. (1996). Wild Mammals in Captivity. Principles & Techniques for Zoo Management. Chicago: USA: University of Chicago Press. 219 pp.
Camelo, V. (2014). Studbook Nacional de Jaguar (Panthera onca). Bogotá D.C.: Acopazoa. 34 pp.
Caughley, G. (1977). Analysis of vertebrate populations. Chichester, United Kingdom: New York: John Wiley and Sons. 234 pp
Drury, H., Hill S. & Saccheri I. (2005). Investigation into inbreeding en European Zoo Population of jaguars (Panthera onca). Zoo Research News. BIAZA Research Newsletter, 6 (4).
Eizirik, E., Yuhki, N., Johnson, W.E., Menotti-Raymond, M., Hannah, S.S. & O'Brien, S.J. (2003). Molecular genetics and evolution of melanism in the cat family. Current Biology, 13(5), 448-453.
Flesness, N. R. (2003). International Species Information System (ISIS): Over 25 years of compiling global animal data to facilitate collection and population management. International Zoo Yearbook, 38, 53-61.
Goodnight, K.F. (2004). Kinship 1.2. User Manual.
Hampson, M.C. & Schwitzer, C. (2016). Effects of Hand-Rearing on Reproductive Success in Captive Large Cats Panthera tigris altaica, Uncia uncia, Acinonyx jubatus and Neofelis nebulosa. PLoS One, 11(5).
Henkel, J.R., Jones, K.L., Hereford, S.G., Savoie, M.L., Leibo, S.P. & Howard, J.J. (2012). Integrating microsatellite and pedigree analyses to facilitate the captive management of the endangered Mississippi sandhill crane (Grus canadensis pulla). Zoo Biology, 31(3).
International species information system (ISIS). (2005). User manual for SPARKS single population analysis and records keeping system.
Jiménez, S. & Moreno. W. (2004). Manejo y anestesia en cinco especies de félidos en cautiverio en parques zoológicos de Colombia. Revista UDCA, Actualidad y Divulgación Científica, 6, 73–83.
Jiménez, S. (2017). Caracterización demográfica, genética y reproductiva de jaguares cautivos (Panthera onca) en cautiverio en parques zoológicos de Colombia. (Trabajo de grado). Bogotá D. C.: Universidad Nacional de Colombia, Facultad de medicina veterinaria y zootecnia, Departamento de salud animal. 129 pp.
Jiménez, S., Ruiz-García, M., Maldonado, J., Jiménez, L., Sánchez, A., Wilbert, T., . . . Jiménez, C. (2017a). Genetic Characterization of Jaguars (Panthera onca) in Captivity in Zoological Parks of Colombia. En ShrivastavA.B & Singh KP (Eds.). Big Cats. 73-91 pp. Rijeka Croacia. Intech.
Jiménez, S., Howard, J. G., Brown, J., Grajales, H., Pinzón, J., Monsalve, H., . . . Jimenez Escobar, C. (2017b). Reproductive analysis of male and female captive jaguars (Panthera onca) in a Colombian zoological park. Theriogenology, 89, 192-200.
Juffe-Bignoli, D., Brooks, T. M., Butchart, S. H. M., Jenkins, R. B., Boe, K., Hoffmann, M., . . . Kingston, N. (2016). Assessing the Cost of Global Biodiversity and Conservation Knowledge. PLoS One, 11(8), e0160640.
Lacy, R. C. (2013). Achieving True Sustainability of Zoo Populations. Zoo Biology, 32(1), 19-26.
Leus, K., Traylor-Holzer, K. & Lacy, R.C. (2011). Genetic and demographic population management in zoos and aquariums: recent developments, future challenges and opportunities for scientific research. International Zoo Yearbook, 45(1), 213-225.
Li, M. H., Strandén, I. & Kantanen, J. (2009). Genetic diversity and pedigree analysis of the Finnsheep breed. Journal of animal science, 87(5), 1598-605.
Lockyear, K.M., Waddell, W.T., Goodrowe, K.L. & MacDonald, S. E. (2009). Retrospective investigation of captive red wolf reproductive success in relation to age and inbreeding. Zoo Biol, 28(3), 214-229.
Menotti-Raymond, M. & O'Brien, S.J. (1995). Evolutionary conservation of ten microsatellite loci in four species of Felidae. Journal of Heredity, 86(4), 319-22.
Pollak, J. P., Lacy R. C. & Ballou J. D. (2005). Population Management 2000, version 1.213. Brookfield, IL.: Chicago Zoological Society.
Seidensticker, L. & Lumpkin, S. (1992). Felinos. Barcelona: Plaza y Janes. Colección Materia viva. 240 pp.
Rueda-Zozaya, P., Mendoza-Martínez, G. D., Martínez-Gómez, D., Monroy-Vilchis, O., Godoy, J. A., Sunny, A., . . . Herrera-Haro, J. (2016). Genetic variability and structure of jaguar (Panthera onca) in Mexican zoos. Genetica, 144(1), 59-69.
Ruiz-García, M., Payán, E., Murillo, A., Álvarez, D. (2006). DNA Microsatellite characterization of the Jaguar (Panthera onca) in Colombia. Genes and Genetics Systems, 81, 115-127.
Zarrate-Charry, D., Laverde Trujillo, L., Balaguera-Reina, S., González-Maya, J. & Trujillo, F. (2010). Rescate y manejo de fauna silvestre ex situ en Colombia: Estudio de caso de un jaguar (Panthera onca) en la Orinoquía Colombiana.CES Medicina Veterinaria y Zootecnia, 4(1), 81-89.
Cómo citar
Las obras publicadas en las revistas del Instituto de Investigación de Recursos Biológicos Alexander von Humboldt están sujetas a los siguientes términos, con relación al derecho de autor:
1. Los derechos patrimoniales de las obras publicadas tienen como titular al Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. Los autores o las instituciones que elaboran el documento aceptan ceder los derechos patrimoniales al Instituto Humboldt con el envío de sus artículos, lo que permite –entre otras cosas– la reproducción, comunicación pública, difusión y divulgación de las obras.
2. Las obras de ediciones digitales se publican bajo una licencia de Creative Commons Colombia:
Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-SinDerivar 4.0 Internacional.
Atribución – No comercial – Sin Derivar: Esta licencia es la más restrictiva de las seis licencias principales, sólo permite que otros puedan descargar las obras y compartirlas con otras personas, siempre que se reconozca su autoría, pero no se pueden cambiar de ninguna manera ni se pueden utilizar comercialmente.
3. Los autores, al someter artículos al proceso editorial de las revistas editadas por el Instituto Humboldt, aceptan las disposiciones institucionales sobre derechos de autor y acceso abierto.
4. Todos los artículos recibidos serán sometidos a un software antiplagio. El sometimiento de un artículo a las revistas del Instituto Humboldt se entiende como la aceptación de la revisión para detectar posible plagio.
5. Las obras sometidas al proceso de edición de las revistas del Instituto Humboldt deben ser inéditas.